INTRODUCTION
The prevalence of atrial fibrillation (AF) increases with age
1 and the risk of stroke similarly rises with aging.
2 Therefore, oral anticoagulation is indicated in elderly patients with AF. Warfarin reduces the risk of stroke but carries an increased bleeding risk, especially in elderly and Asian patients.
3 Non-vitamin K antagonist oral anticoagulants (NOACs) proved safety and efficacy compared to warfarin overall age.
4
The Rivaroxaban Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) study proved the non-inferiority of rivaroxaban compared to warfarin in terms of efficacy and safety.
5 During subgroup analysis in the ROCKET AF trial according to the age, elderly patients showed similar results for the efficacy and safety of rivaroxaban relative to warfarin. However, the absolute event rate was higher than that in younger patients, especially considering major bleeding events.
6 Patients in Japan were not enrolled in the global ROCKET AF trial and participated instead in the Japanese Rivaroxaban Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation (J-ROCKET AF) study according to the pharmacokinetic modeling data.
7 J-ROCKET AF compared the efficacy and safety of rivaroxaban 15 mg once daily (10 mg once daily in patients with creatinine clearance [CrCl] < 50 mL/min) and warfarin in Japanese patients. The J-ROCKET AF study ultimately showed the same efficacy endpoint as the global ROCKET AF study and proved the non-inferiority of rivaroxaban in safety outcomes compared to warfarin. According to this result, Japan and Taiwan approved a low-dose rivaroxaban regimen (LDRR, 15/10 mg) for stroke prevention in patients with AF.
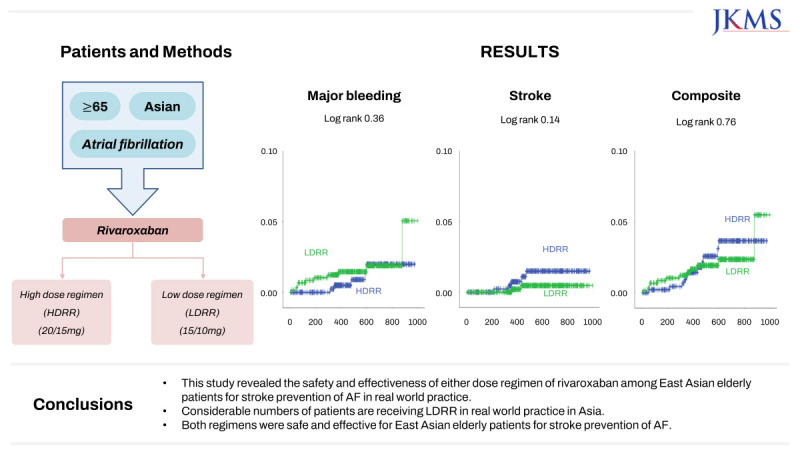
In this study, we aimed to assess the safety and efficacy of a high-dose rivaroxaban regimen (HDRR, 20/15 mg) and LDRR among elderly East Asian patients with AF in real-world practice.
METHODS
Study population
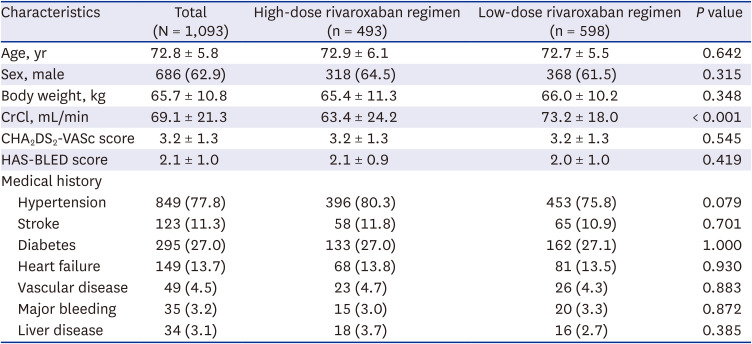
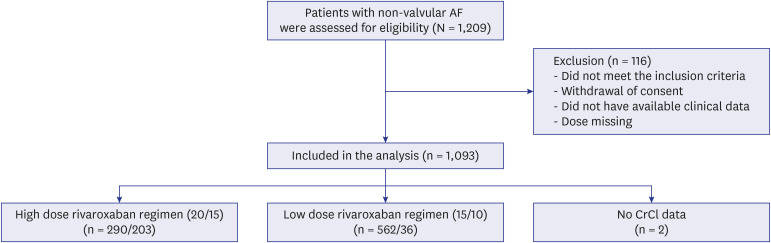
The Rivaroxaban in Elderly NVAF Patients with or without Renal Impairment (XAIENT) study is a prospective, non-interventional, multicenter observational study performed to evaluate the safety and efficacy of rivaroxaban in patients with AF among elderly East Asian patients. Patients > 65 years of age with non-valvular AF indicated to receive oral anticoagulation for stroke prevention with a CHA2DS2-VASc score more than 2 and prescribed any dose of rivaroxaban were included from October 2019 to April 2021. We excluded patients who had a history of mechanical valve surgery or moderate to severe mitral stenosis. Those patients who did not meet the inclusion criteria, did not have available clinical data, or did not have dose information were excluded from analysis.
Data collection and study outcomes
The globally recommended label was rivaroxaban 20 mg once daily for patients with CrCl ≥ 50 mL/min and rivaroxaban 15 mg once daily for patients with CrCl < 50 mL/min. In Japan, the approved prescription is rivaroxaban 15 mg once daily for patients with CrCl ≥ 50 mL/min and 10 mg once daily for patients with CrCl < 50 mL/min. In our registry, the prescription dose of rivaroxaban was dependent on the physician’s discretion. Therefore, patients were divided into two groups according to the global label (HDRR) and the Japan label (LDRR). The basic demographic data of each participant was acquired from a database, including age, sex, body weight, renal function, and other medical histories. The clinical endpoints include major bleeding, ischemic stroke, and a composite of ischemic stroke or major bleeding or all-cause mortality. Major bleeding was defined by the International Society of Thrombosis and Haemostasis criteria including fatal bleeding, critical organ bleeding, or bleeding causing a fall in hemoglobin level of 2 g/dL or more.
8 Data were collected at baseline and during a follow-up period of ≥ 12 months.
Statistical analysis
The baseline characteristics are presented as mean ± standard deviation values for continuous variables and as frequencies with percentages for categorical variables. Continuous variables were compared using an unpaired t test, and categorical variables were compared using either the χ2 test or Fisher’s exact test as appropriate. Event rate curves were obtained by Kaplan–Meier analysis and compared using the log-rank test. The outcomes were assessed using a Cox proportional hazards model adjusted for age, sex, and renal function and are presented as hazard ratios (HRs). P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 27.0 (IBM Corporation, Armonk, NY, USA).
Ethics statement
This study was approved by the local Institutional Review Board (IRB) of Samsung Medical Center in South Korea (IRB No. SMC 2019-06-078). All patients provided informed consent and were registered at ClinicalTrials.gov (
NCT04096547).
DISCUSSION
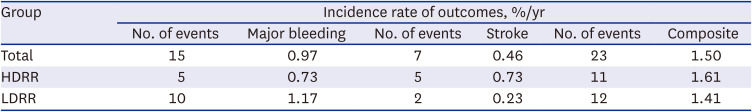
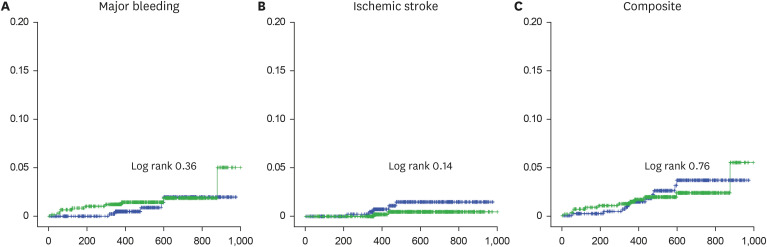
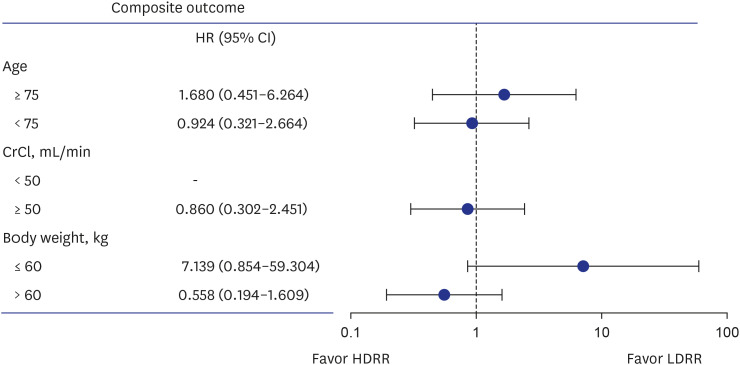
This study was a prospective real-world study designed to evaluate the safety and efficacy of rivaroxaban among elderly East Asian patients for stroke prevention of AF according to various dosing criteria in real-world practice. About 50% of Asian patients receive LDRR in real-world clinical practice. Both regimens carried similar risks of major bleeding, stroke, and the composite outcome. In subgroup analysis according to age, CrCl, and body weight, there were no significant differences in the composite outcome between the regimen groups.
The standard dose criterion for rivaroxaban is 20 mg once daily (or 15 mg once daily in patients with CrCl < 50 mL/min) based on the ROCKET AF trial,
5 while, in the J-ROCKET AF trial, the rivaroxaban dose criteria were 15 mg once daily in patients with CrCl ≥ 50 mL/min and 10 mg once daily in patients with CrCl < 50 mL/min.
7 In Taiwan, either the ROCKET AF or J-ROCKET AF dosage criteria was adopted for stroke prevention, while Korea approved the standard dose criteria for rivaroxaban according to the global recommendation. However, a considerable number of elderly patients (54.7%) in real-world practice in Asia are receiving LDRR therapy. As a result, 70% of patients among the HDRR and LDRR groups were prescribed 15 mg of rivaroxaban. A similar trend was observed in another Korean registry study where the ratio of off-label underdose use of rivaroxaban.
9 There might be several reasons for this trend in clinical practice. First, the ROCKET AF trial proved the efficacy and safety of rivaroxaban compared to warfarin; however, the study included only 6.5% of Asian population. The characteristics of Korean patients are likely more similar to those of the J-ROCKET AF population. Second, there were no body weight criteria for dose reduction of rivaroxaban. Asian patients usually have lower body weights compared to other races. According to our results, the overall mean body weight was 65 kg, and patients receiving the 15-mg LDRR dose tended to be older, female patients with lower body weights and CrCl values compared to those receiving 20 mg in the HDRR group. In other words, patients with a higher bleeding risk or borderline renal function received the 15-mg LDRR dose. Therefore, we should compare the safety and efficacy of both criteria in the Asian population. Chan et al.
10 reported that the J-ROCKET AF dosage was as effective as the ROCKET AF dosage and the risk of major bleeding was lower with the J-ROCKET AF criteria in Taiwanese patients with impaired renal function. This study was a retrospective study, and the J-ROCKET AF criteria group was significantly older. As age is a major risk factor for stroke and bleeding, it is necessary to be wary when interpreting the results. In another study, investigators compared the effectiveness and safety of rivaroxaban 20 mg and 15 mg in patients with preserved renal function (CrCl ≥ 50 mL/min).
11 This was a retrospective analysis study based on Korean National Health Insurance Service data where both doses were associated with reduced stroke and bleeding risk compared to warfarin and the 20-mg dose led to a better composite outcome compared to the 15-mg dose in patients with preserved renal function. According to our prospective real-world study results, there were no significant differences between the HDRR and LDRR groups in stroke, major bleeding, and the composite outcome among elderly East Asian patients regardless of renal function. In this study, we included the patients with impaired renal function (CrCl < 50 mL/min). Furthermore, we included patients > 65 years of age. Therefore, mean age was older in our study compared to previous study. Also, there were no significant differences in the composite outcome according to age, renal function, or body weight.
There are some pharmacokinetics and pharmacodynamics analysis reports concerning rivaroxaban in the literature. Renal function was the only identified relevant factor for exposure–response relationship among factors including body weight, nationality among the countries in Asia, and hepatic function. Furthermore, the clearance of rivaroxaban in Asians was 31–43% lower than that in Caucasians. As a result, a reduced dose of rivaroxaban might be required for Asians.
12 These pharmacokinetics/pharmacodynamics data support our clinical outcomes data according to the different dose regimens. Also, the Xarelto Post-Authorization Safety and Effectiveness Study in Japanese Patients with Atrial Fibrillation (XAPASS) trial, which was the largest single-arm study conducted in Japan, proved that rivaroxaban therapy according to the LDRR criteria is safe and effective in real-world clinical practice (with an event rate of major bleeding of 1.8%/year and event rate of stroke, systemic embolism, and myocardial infarction of 1.8%/year).
13 Therefore, we should consider the potential for ethnic differences when using rivaroxaban.
This study had some limitations. First, it was a non-interventional single-arm observational study. The selection of each dose regimen was also left to the physician’s discretion. Therefore, there might be a potential risk of bias in that the LDRR group was more likely to contain patients at high risk of bleeding. In fact, physicians prefer to prescribe low-dose rivaroxaban to older and frail patients. Nevertheless, there were no significant differences in stroke risk between the groups. In other words, LDRR might be safe without increasing the stroke risk for specific Asian populations. Second, the event rate of outcomes showed a relatively low incidence compared to results in other studies, including randomized controlled trials. Considering that our study did not include myocardial infarction or any bleeding in the outcome, it would be expected to show an event rate similar to that of other real-world studies.
In conclusion, this study revealed the safety and effectiveness of either dose regimen of rivaroxaban among elderly East Asian patients for stroke prevention of AF in real-world practice. Considerable numbers of patients are receiving LDRR in real-world practice in Asia. Both regimens were safe and effective for elderly East Asian patients for stroke prevention of AF.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download