2. Garmendia Madariaga A, Santos Palacios S, Guillén-Grima F, Galofré JC. 2014; The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab. 99:923–931. DOI:
10.1210/jc.2013-2409. PMID:
24423323.

4. Danzi S, Klein I. 2014; Thyroid disease and the cardiovascular system. Endocrinol Metab Clin North Am. 43:517–528. DOI:
10.1016/j.ecl.2014.02.005. PMID:
24891175.

5. Razvi S, Jabbar A, Pingitore A, Danzi S, Biondi B, Klein I, Peeters R, Zaman A, Iervasi G. 2018; Thyroid hormones and cardiovascular function and diseases. J Am Coll Cardiol. 71:1781–1796. DOI:
10.1016/j.jacc.2018.02.045. PMID:
29673469.

6. Bektur Aykanat NE, Şahin E, Kaçar S, Bağcı R, Karakaya Ş, Burukoğlu Dönmez D, Şahintürk V. 2021; Cardiac hypertrophy caused by hyperthyroidism in rats: the role of ATF-6 and TRPC1 channels. Can J Physiol Pharmacol. 99:1226–1233. DOI:
10.1139/cjpp-2021-0260. PMID:
34283935.

7. Wang YY, Jiao B, Guo WG, Che HL, Yu ZB. 2010; Excessive thyroxine enhances susceptibility to apoptosis and decreases contractility of cardiomyocytes. Mol Cell Endocrinol. 320:67–75. DOI:
10.1016/j.mce.2010.01.031. PMID:
20122986.
9. Freitas F, Estato V, Carvalho VF, Torres RC, Lessa MA, Tibiriçá E. 2013; Cardiac microvascular rarefaction in hyperthyroidism-induced left ventricle dysfunction. Microcirculation. 20:590–598. DOI:
10.1111/micc.12057. PMID:
23510303.

11. Yue WS, Chong BH, Zhang XH, Liao SY, Jim MH, Kung AW, Tse HF, Siu CW. 2011; Hyperthyroidism-induced left ventricular diastolic dysfunction: implication in hyperthyroidism-related heart failure. Clin Endocrinol (Oxf). 74:636–643. DOI:
10.1111/j.1365-2265.2011.03981.x. PMID:
21470287.
12. Wang YY, Morimoto S, Du CK, Lu QW, Zhan DY, Tsutsumi T, Ide T, Miwa Y, Takahashi-Yanaga F, Sasaguri T. 2010; Up-regulation of type 2 iodothyronine deiodinase in dilated cardiomyopathy. Cardiovasc Res. 87:636–646. DOI:
10.1093/cvr/cvq133. PMID:
20453157.
14. Kumar A, Sinha RA, Tiwari M, Singh R, Koji T, Manhas N, Rastogi L, Pal L, Shrivastava A, Sahu RP, Godbole MM. 2007; Hyperthyroidism induces apoptosis in rat liver through activation of death receptor-mediated pathways. J Hepatol. 46:888–898. DOI:
10.1016/j.jhep.2006.12.015. PMID:
17321637.
15. Hu H, Tian M, Ding C, Yu S. 2019; The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front Immunol. 9:3083. DOI:
10.3389/fimmu.2018.03083. PMID:
30662442. PMCID:
PMC6328441.
17. Li Y, Guo Y, Tang J, Jiang J, Chen Z. 2015; New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai). 47:146–147. Erratum for:
Acta Biochim Biophys Sin (Shanghai). 2014;46:629-640. DOI:
10.1093/abbs/gmu048. PMID:
25016584.
19. He L, Yuan L, Yu W, Sun Y, Jiang D, Wang X, Feng X, Wang Z, Xu J, Yang R, Zhang W, Feng H, Chen HZ, Zeng YA, Hui L, Wu Q, Zhang Y, Zhang L. 2020; A regulation loop between YAP and NR4A1 balances cell proliferation and apoptosis. Cell Rep. 33:108284. DOI:
10.1016/j.celrep.2020.108284. PMID:
33086070.
20. Zhang J, Liu J, Gao S, Lin W, Gao P, Gao K, Zhang Y, Du K, Yang X, Wang W, Zhu R, Wang Y. 2019; Antiapoptosis and antifibrosis effects of
Qishen granules on heart failure rats via Hippo pathway. Biomed Res Int. 2019:1642575. Erratum in:
Biomed Res Int. 2020;2020: 8569251. DOI:
10.1155/2019/1642575. PMID:
31915683. PMCID:
PMC6930732.
21. Wu H, Wei L, Fan F, Ji S, Zhang S, Geng J, Hong L, Fan X, Chen Q, Tian J, Jiang M, Sun X, Jin C, Yin ZY, Liu Q, Zhang J, Qin F, Lin KH, Yu JS, Deng X, et al. 2015; Integration of Hippo signalling and the unfolded protein response to restrain liver overgrowth and tumorigenesis. Nat Commun. 6:6239. DOI:
10.1038/ncomms7239. PMID:
25695629.
22. Ashokkumar R, Jamuna S, Sakeena Sadullah MS, Niranjali Devaraj S. 2018; Vitexin protects isoproterenol induced post myocardial injury by modulating hipposignaling and ER stress responses. Biochem Biophys Res Commun. 496:731–737. DOI:
10.1016/j.bbrc.2018.01.104. PMID:
29406244.
23. Ouyang H, Zhong J, Lu J, Zhong Y, Hu Y, Tan Y. 2019; Inhibitory effect of melatonin on Mst1 ameliorates myocarditis through attenuating ER stress and mitochondrial dysfunction. J Mol Histol. 50:405–415. DOI:
10.1007/s10735-019-09836-w. PMID:
31256303.
24. Huang Y, Tang C, Du J, Jin H. 2016; Endogenous sulfur dioxide: a new member of gasotransmitter family in the cardiovascular system. Oxid Med Cell Longev. 2016:8961951. DOI:
10.1155/2016/8961951. PMID:
26839635. PMCID:
PMC4709694.
25. Wang XB, Cui H, Du JB. 2019; Potential therapeutic effect of SO₂ on fibrosis. Histol Histopathol. 34:1289–1297.
26. Hwang JH, Kang SY, Kang AN, Jung HW, Jung C, Jeong JH, Park YK. 2017; MOK, a pharmacopuncture medicine, regulates thyroid dysfunction in L-thyroxin-induced hyperthyroidism in rats through the regulation of oxidation and the TRPV1 ion channel. BMC Complement Altern Med. 17:535. DOI:
10.1186/s12906-017-2036-1. PMID:
29246135. PMCID:
PMC5732465.
27. Yu W, Liu D, Liang C, Ochs T, Chen S, Chen S, Du S, Tang C, Huang Y, Du J, Jin H. 2016; Sulfur dioxide protects against collagen accumulation in pulmonary artery in association with downregulation of the transforming growth factor β1/Smad pathway in pulmonary hypertensive rats. J Am Heart Assoc. 5:e003910. DOI:
10.1161/JAHA.116.003910. PMID:
27792648. PMCID:
PMC5121494.
28. Liu M, Liu S, Tan W, Tang F, Long J, Li Z, Liang B, Chu C, Yang J. 2017; Gaseous signalling molecule SO
2 via HippoMST pathway to improve myocardial fibrosis of diabetic rats. Mol Med Rep. 16:8953–8963. DOI:
10.3892/mmr.2017.7714. PMID:
28990064. PMCID:
PMC5779980.

29. Bao M, Hua X, Mo H, Sun Z, Xu B, Chen X, Xu M, Xu X, Song J. 2022; N-acetylcysteine, an ROS inhibitor, alleviates the pathophysiology of hyperthyroidism-induced cardiomyopathy via the ROS/Ca
2+ pathway. Biomolecules. 12:1195. DOI:
10.3390/biom12091195. PMID:
36139036. PMCID:
PMC9496499.
30. Lv B, Peng H, Qiu B, Zhang L, Ge M, Bu D, Li K, Yu X, Du J, Yang L, Tang C, Huang Y, Du J, Jin H. 2022; Sulphenylation of CypD at cysteine 104: a novel mechanism by which SO
2 inhibits cardiomyocyte apoptosis. Front Cell Dev Biol. 9:784799. DOI:
10.3389/fcell.2021.784799. PMID:
35118072. PMCID:
PMC8805922.
31. Zhang D, Wang X, Tian X, Zhang L, Yang G, Tao Y, Liang C, Li K, Yu X, Tang X, Tang C, Zhou J, Kong W, Du J, Huang Y, Jin H. 2018; The increased endogenous sulfur dioxide acts as a compensatory mechanism for the downregulated endogenous hydrogen sulfide pathway in the endothelial cell inflammation. Front Immunol. 9:882. DOI:
10.3389/fimmu.2018.00882. PMID:
29760703. PMCID:
PMC5936987.
34. Khamis T, Alsemeh AE, Abdullah DM. 2022; Sacubitril/valsartan (LCZ696) ameliorates hyperthyroid-induced cardiac hypertrophy in male rats through modulation of miR-377, let-7 b, autophagy, and fibrotic signaling pathways. Sci Rep. 12:14654. DOI:
10.1038/s41598-022-18860-y. PMID:
36030321. PMCID:
PMC9420135.

38. Li YY, McTiernan CF, Feldman AM. 2000; Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res. 46:214–224. DOI:
10.1016/S0008-6363(00)00003-1. PMID:
10773225.
39. Foster CR, Daniel LL, Daniels CR, Dalal S, Singh M, Singh K. 2013; Deficiency of ataxia telangiectasia mutated kinase modulates cardiac remodeling following myocardial infarction: involvement in fibrosis and apoptosis. PLoS One. 8:e83513. DOI:
10.1371/journal.pone.0083513. PMID:
24358288. PMCID:
PMC3865210.

40. Jiang M, Qi L, Li L, Li Y. 2020; The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 6:112. DOI:
10.1038/s41420-020-00349-0. PMID:
33133646. PMCID:
PMC7595122.

41. Boudreau MW, Peh J, Hergenrother PJ. 2019; Procaspase-3 overexpression in cancer: a paradoxical observation with therapeutic potential. ACS Chem Biol. 14:2335–2348. DOI:
10.1021/acschembio.9b00338. PMID:
31260254. PMCID:
PMC6858495.
42. Czabotar PE, Lessene G, Strasser A, Adams JM. 2014; Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 15:49–63. DOI:
10.1038/nrm3722. PMID:
24355989.

43. Teixeira RB, Barboza TE, DE Araujo CC, Siqueira R, DE Castro AL, Bonetto JHP, DE Lima-Seolin BG, Carraro CC, Bello-Klein A, Singal PK, Araujo ASDR. 2018; Decreased PGC1-α levels and increased apoptotic protein signaling are associated with the maladaptive cardiac hypertrophy in hyperthyroidism. J Biosci. 43:887–895. DOI:
10.1007/s12038-018-9816-8. PMID:
30541949.
44. Liu MQ, Chen Z, Chen LX. 2016; Endoplasmic reticulum stress: a novel mechanism and therapeutic target for cardiovascular diseases. Acta Pharmacol Sin. 37:425–443. DOI:
10.1038/aps.2015.145. PMID:
26838072. PMCID:
PMC4820795.

45. Bravo R, Gutierrez T, Paredes F, Gatica D, Rodriguez AE, Pedrozo Z, Chiong M, Parra V, Quest AF, Rothermel BA, Lavandero S. 2012; Endoplasmic reticulum: ER stress regulates mitochondrial bioenergetics. Int J Biochem Cell Biol. 44:16–20. DOI:
10.1016/j.biocel.2011.10.012. PMID:
22064245. PMCID:
PMC4118286.

46. Shi Y, Wang S, Peng H, Lv Y, Li W, Cheng S, Liu J. 2019; Fibroblast growth factor 21 attenuates vascular calcification by alleviating endoplasmic reticulum stress mediated apoptosis in rats. Int J Biol Sci. 15:138–147. DOI:
10.7150/ijbs.28873. PMID:
30662354. PMCID:
PMC6329919.

48. Luo T, Kim JK, Chen B, Abdel-Latif A, Kitakaze M, Yan L. 2015; Attenuation of ER stress prevents post-infarction-induced cardiac rupture and remodeling by modulating both cardiac apoptosis and fibrosis. Chem Biol Interact. 225:90–98. DOI:
10.1016/j.cbi.2014.10.032. PMID:
25450231. PMCID:
PMC4684183.
49. Hamada H, Suzuki M, Yuasa S, Mimura N, Shinozuka N, Takada Y, Suzuki M, Nishino T, Nakaya H, Koseki H, Aoe T. 2004; Dilated cardiomyopathy caused by aberrant endoplasmic reticulum quality control in mutant KDEL receptor transgenic mice. Mol Cell Biol. 24:8007–8017. DOI:
10.1128/MCB.24.18.8007-8017.2004. PMID:
15340063. PMCID:
PMC515036.
50. Meng S, Zhang W, Guan LJ, Muhali FS, Zhou JZ, Song RH, Xu J, Zhang JA. 2017; Proteomic analysis reveals aberrant expression of CALR and HSPA5 in thyroid tissues of Graves' disease. Clin Biochem. 50:40–45. DOI:
10.1016/j.clinbiochem.2016.08.014. PMID:
27566407.

53. Deshpande A, Borlepawar A, Rosskopf A, Frank D, Frey N, Rangrez AY. 2021; SH3-binding glutamic acid rich-deficiency augments apoptosis in neonatal rat cardiomyocytes. Int J Mol Sci. 22:11042. DOI:
10.3390/ijms222011042. PMID:
34681711. PMCID:
PMC8541172.
54. Francisco J, Zhang Y, Jeong JI, Mizushima W, Ikeda S, Ivessa A, Oka S, Zhai P, Tallquist MD, Del Re DP. 2020; Blockade of fibroblast YAP attenuates cardiac fibrosis and dysfunction through MRTF-A inhibition. JACC Basic Transl Sci. 5:931–945. Erratum in:
JACC Basic Transl Sci. 2021;6:629. DOI:
10.1016/j.jacbts.2020.07.009. PMID:
33015415. PMCID:
PMC7524792.
55. Zhang M, Zhang L, Hu J, Lin J, Wang T, Duan Y, Man W, Feng J, Sun L, Jia H, Li C, Zhang R, Wang H, Sun D. 2016; MST1 coordinately regulates autophagy and apoptosis in diabetic cardiomyopathy in mice. Diabetologia. 59:2435–2447. DOI:
10.1007/s00125-016-4070-9. PMID:
27510910.
56. Su D, Li Y, Guan L, Li Q, Shi C, Ma X, Song Y. 2021; Elevated MST1 leads to apoptosis via depletion of YAP1 in cardiomyocytes exposed to high glucose. Mol Med. 27:13. DOI:
10.1186/s10020-021-00267-6. PMID:
33568044. PMCID:
PMC7874454.

57. Huang Y, Zhang H, Lv B, Tang C, Du J, Jin H. 2022; Sulfur dioxide: endogenous generation, biological effects, detection, and therapeutic potential. Antioxid Redox Signal. 36:256–274. DOI:
10.1089/ars.2021.0213. PMID:
34538110.
58. Liu J, Huang Y, Chen S, Tang C, Jin H, Du J. 2016; Role of endogenous sulfur dioxide in regulating vascular structural remodeling in hypertension. Oxid Med Cell Longev. 2016:4529060. DOI:
10.1155/2016/4529060. PMID:
27721913. PMCID:
PMC5046050.
59. Jin H, Liu AD, Holmberg L, Zhao M, Chen S, Yang J, Sun Y, Chen S, Tang C, Du J. 2013; The role of sulfur dioxide in the regulation of mitochondrion-related cardiomyocyte apoptosis in rats with isopropylarterenol-induced myocardial injury. Int J Mol Sci. 14:10465–10482. DOI:
10.3390/ijms140510465. PMID:
23698774. PMCID:
PMC3676849.
60. Zhao J, Wu Q, Yang T, Nie L, Liu S, Zhou J, Chen J, Jiang Z, Xiao T, Yang J, Chu C. 2022; Gaseous signal molecule SO
2 regulates autophagy through PI3K/AKT pathway inhibits cardiomyocyte apoptosis and improves myocardial fibrosis in rats with type II diabetes. Korean J Physiol Pharmacol. 26:541–556. DOI:
10.4196/kjpp.2022.26.6.541. PMID:
36302628. PMCID:
PMC9614393.

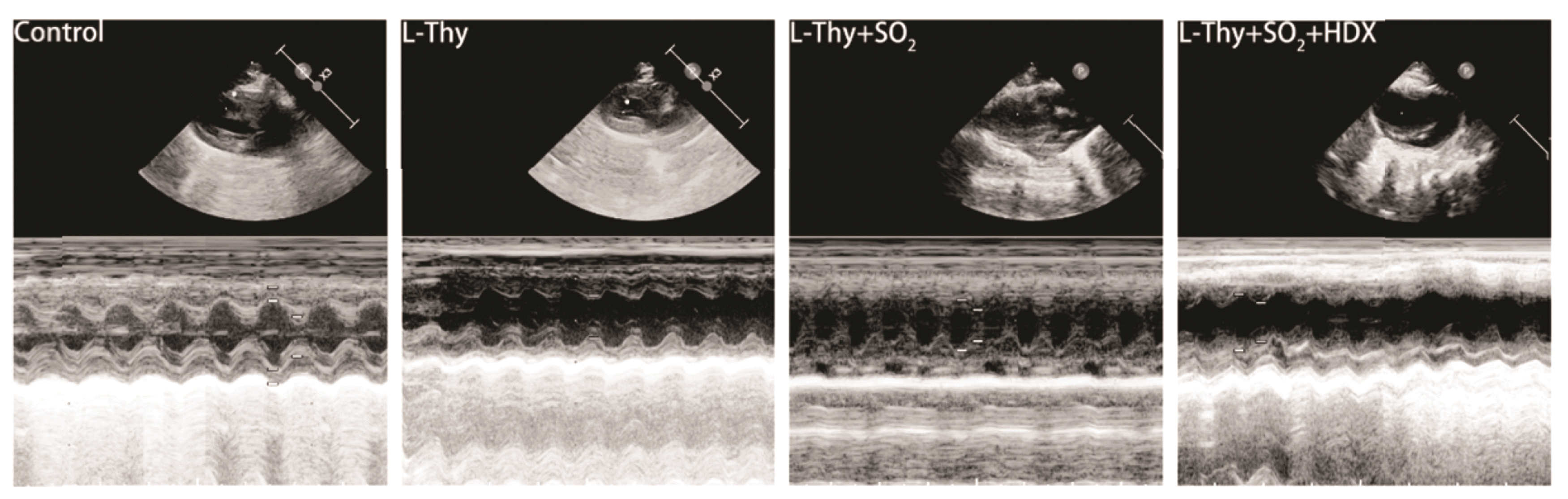








 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download