Abstract
Vancomycin is a frequently used antibiotic in intensive care units, and the patient’s renal clearance affects the pharmacokinetic characteristics of vancomycin. Several advantages have been reported for vancomycin continuous intravenous infusion, but studies on continuous dosing regimens based on patients’ renal clearance are insufficient. The aim of this study was to develop a vancomycin serum concentration prediction model by factoring in a patient’s renal clearance. Children admitted to our institution between July 1, 2021, and July 31, 2022 with records of continuous infusion of vancomycin were included in the study. Sex, age, height, weight, vancomycin dose by weight, interval from the start of vancomycin administration to the time of therapeutic drug monitoring sampling, and vancomycin serum concentrations were analyzed with the linear regression analysis of the mixed effect model. Univariable regression analysis was performed using the vancomycin serum concentration as a dependent variable. It showed that vancomycin dose (p < 0.001) and serum creatinine (p = 0.007) were factors that had the most impact on vancomycin serum concentration. Vancomycin serum concentration was affected by vancomycin dose (p < 0.001) and serum creatinine (p = 0.001) with statistical significance, and a multivariable regression model was obtained as follows: Vancomycin serum concentration (mg/l) = –1.296 + 0.281 × vancomycin dose (mg/kg) + 20.458 × serum creatinine (mg/dl) (adjusted coefficient of determination, R2 = 0.66). This prediction model is expected to contribute to establishing an optimal continuous infusion regimen for vancomycin.
Vancomycin is one of the most frequently used antibiotics in intensive care units (ICU) for methicillin-resistant Staphylococcus aureus (MRSA) infection [1]. Initial vancomycin dosing for the treatment of children with MRSA bacteremia or infective endocarditis is usually 15 mg/kg/dose intravenously every 6 h [1]. The dosing needs to be adjusted on the basis of trough and peak serum concentrations [1]. Continuous intravenous (CIV) infusion of vancomycin may be an alternative administration method. Previous studies suggest that CIV infusion can be more beneficial over intermittent intravenous (IIV) infusion. This is because the therapeutic level of vancomycin could be reached more quickly than IIV infusion, carries a lower risk of nephrotoxicity, and requires a smaller number of samplings for monitoring [2,3]. However, current consensus guidelines lack recommendations on vancomycin CIV dosing for pediatric patients.
Several studies in the adult population have explored the optimization of vancomycin CIV administration [2-6]. Although their results and recommendations varied, these studies unanimously agreed that renal clearance is a crucial factor in titrating the dosage of vancomycin CIV infusion [2-6]. Hong et al. [4] administered vancomycin CIV infusion using a dosing regimen with loading doses of 20 mg/kg and 15–40 mg/kg as a maintenance dose based on the patient’s serum creatinine. Roberts et al. [5] suggested administering vancomycin at 35 mg/kg for 3 h as a loading dose and 35 mg/kg as a maintenance dose with adjustments according to the patient’s renal clearance. Similar to the study by Roberts et al. [5], Cristallini et al. [6] suggested 35 mg/kg as a loading dose and 7–45 mg/kg as a maintenance dose, depending on the patient’s renal clearance.
Although previous studies on adults concluded that the renal clearance of the patient has a great influence on the pharmacokinetic (PK) characteristics of vancomycin [7], vancomycin CIV dosing considering renal clearance has not been established in children. It is necessary to establish renal dosing of vancomycin CIV administration based on renal clearance in pediatric patients, particularly critically ill pediatric patients who are admitted to the pediatric intensive care unit (PICU), because they generally have a wider range of renal clearance, from patients with augmented renal clearance, in which drug elimination is increased [8], to patients with reduced renal clearance due to acute kidney injury. Several retrospective studies have been conducted on pediatric populations to determine the optimal CIV dosage for achieving therapeutic vancomycin serum concentrations. However, none of these studies considered renal clearance [9-14]. There are 2 studies done on neonates where serum creatinine was considered for determining the CIV dosage of vancomycin [15,16], yet it is difficult to apply the dosing regimen of neonates to older children due to differences in PK parameters such as volume of distribution, half-life, and drug clearance [17-19].
With studies on vancomycin CIV dosing based on renal clearance for critically ill children lacking, our aim was to develop a prediction model of vancomycin serum concentration considering a patient’s renal clearance. Accurate prediction of vancomycin serum concentration is necessary to minimize subtherapeutic or toxic exposure [3]. Ultimately, this model would help determine the optimal vancomycin CIV dosing regimen for pediatric patients.
This retrospective study was carried out at a tertiary teaching hospital based in Seoul, Republic of Korea. Pediatric patients under the age of 18 who were admitted to the PICU between July 1, 2021, and July 31, 2022 with records of vancomycin CIV administration and therapeutic drug monitoring (TDM) were included in the study.
Exclusion criteria were: (i) cases in which vancomycin serum concentration did not reach steady state; (ii) cases in which blood samples were collected within 16 h after the start of administration; (iii) cases with a difference in sampling time of more than 24 h between serum creatinine and vancomycin serum concentration; (iv) patients diagnosed with neuromuscular disease that could affect serum creatinine levels; and (v) patients on continuous renal replacement therapy (CRRT) or patients with extracorporeal membrane oxygenation (ECMO) therapy. Cases not reaching a steady state were defined as those failing to reach a plateau when plotting a time-vancomycin serum concentration graph using the Abbott base PK system version 1.10 (DOSBox version 0.74). The samples were drawn at least 16 h after the start of administration to ensure that the serum level of vancomycin has reached a plateau.
Data were obtained through the electronic medical record system of the hospital. Basic information such as the patient’s age, sex, height, weight, body surface area (BSA), body mass index (BMI), underlying disease, and CRRT/ECMO application status was collected. Data needed for determining the PKs of vancomycin CIV infusion, including the total daily amount of vancomycin infused, the start date and duration of vancomycin therapy, the serum concentration of vancomycin along with its sampling time, and laboratory test results reflecting a patient’s renal clearance such as serum creatinine and cystatin C, were also collected.
In accordance with the hospital protocol, vancomycin was administered as CIV infusion at 15 mg/kg over 1 h as a loading dose, followed by 45 mg/kg as a maintenance dose. The target serum concentration was 20–25 mg/l regardless of indication [2]. The sampling time for checking vancomycin serum concentration was 18–30 h after the start of administration. As the trough level could not be determined during CIV infusion [20], we considered the daily dosing of vancomycin therapeutic as long as the vancomycin serum concentration remained within the range of 20–25 mg/l. Follow-up TDM was conducted every 48 h or in the event of a significant change in the patient’s condition. The dosing of vancomycin was adjusted to meet the target serum concentration of 20–25 mg/l.
The initial objective of this study was to identify the factors that may affect vancomycin serum concentration in critically ill pediatric patients. Employing these factors, a prediction model of vancomycin serum concentration was derived as the primary outcome.
To prevent the undue influence of multiple data points from a single patient, we employed a mixed-effects model for our statistical analysis [21]. The correlation between each factor and the vancomycin serum concentration was analyzed using the univariate mixed-effects model regression analysis, with patients as random variables and vancomycin serum concentration as fixed variable to identify factors that could affect vancomycin serum concentration. Factors identified to be correlated with vancomycin serum concentration were included in the multivariate regression analysis of the mixed-effects model to derive an equation for the vancomycin dosage according to a patient’s serum creatinine and target serum concentration. R software version 4.2.1 (R Project for Statistical Computing) was used for data analysis, and a p-value < 0.05 was evaluated as statistically significant. Continuous data are presented as medians (interquartile range), and categorical data are presented as numbers (%).
Among 477 cases (86 patients) of vancomycin CIV administration with vancomycin TDM, a total of 103 cases (29 patients) met the inclusion criteria. The selection process for the cases is shown in Fig. 1. The median age of patients was 13 (6.5–50.5) months, and patients with underlying respiratory disease were the most common at 41 patients (39.8%). The median value of the vancomycin dose was 45 (33.2–68.8) mg/kg/day, and the vancomycin serum concentration was 22.1 (18.3–24.6) mg/l. The median serum creatinine of patients included in the study was 0.36 (0.31–0.46) mg/dl (Table 1).
A univariate regression analysis of the mixed effect model was performed for each of the 103 cases, with vancomycin serum concentration as a dependent variable and the patient’s age, sex, height, weight, BSA, BMI, vancomycin dose, Tsample (time between the start of vancomycin administration and TDM sample collection), and serum creatinine as an independent variable. The analysis showed that vancomycin dose (p < 0.001) and serum creatinine (p = 0.007) were 2 factors that affected the serum concentration of vancomycin (Table 2). Renal clearance did not have a statistically significant impact on the vancomycin serum concentration.
Based on the results of the univariate regression analysis of the mixed effect model, the multivariate regression analysis of the mixed effect model was done. The vancomycin serum concentration was employed as the dependent variable, and the vancomycin dose and serum creatinine were employed as independent variables (Table 2). The analysis showed that both factors had statistically significant relationships with the vancomycin serum concentration (p-values were < 0.001 and 0.001, respectively). A multivariate regression model was derived as follows (adjusted coefficient of determination, R2 = 0.66):
Vancomycin serum concentration (mg/l)
= –1.296 + 0.281 × vancomycin dose (mg/kg) + 20.458 × serum creatinine (mg/dl)
The median value of the predicted vancomycin serum concentrations for the study population was 21.2 (17.1–25.1) mg/l (Fig. 2).
This study validates the high impact of renal clearance, reflected by serum creatinine, on vancomycin serum concentration, consistent with multiple studies [2-6]. With this finding, we derived a multivariate regression model for the prediction of vancomycin serum concentration during CIV infusion. The median value of the predicted vancomycin serum concentrations for the study population was 21.2 (17.1–25.1) mg/l, comparable to the observed vancomycin serum concentration of 22.1 (18.3–24.6) mg/l (Fig. 2). Despite the relatively low coefficient of determination at R2 = 0.66, we can conclude that serum creatinine, available in routine lab workup, can also be used to predict vancomycin serum concentration. It would be beneficial to use a readily available lab value in rapidly changing clinical settings such as PICU because TDM can only be done in regular working hours, and it takes time to obtain the results. To our knowledge, this is the first attempt to predict the steady-state serum concentration of vancomycin rather than the trough vancomycin concentration.
Vancomycin is a potent antibiotic agent against Gram-positive bacteria that inhibits the synthesis of the bacterial cell wall. It is regarded as the first-line treatment for infections caused by MRSA [22]. The minimal inhibitory concentration (MIC) of the pathogen and the risk of nephrotoxicity are 2 major components that govern the optimal dosing of vancomycin. In order to determine the efficacy of vancomycin in terms of MIC, serum trough levels are measured for the titration of the traditional IIV infusion method. However, the risk of nephrotoxicity increases when the serum trough level of vancomycin is maintained at a high level [22]. Therefore, CIV infusion of vancomycin has been suggested as an alternative approach for increasing the efficacy of vancomycin and reducing unwanted nephrotoxicity [22], and in some parts of the world, including some European countries such as Germany, Italy, and Belgium, vancomycin is already employing CIV infusion instead of IIV infusion as a routine method of administration in the ICU setting [23-25].
The advantages of vancomycin CIV administration are highlighted in several other studies. In a retrospective cohort study of adult burn patients admitted to the ICU, the incidence of reaching therapeutic range was higher at 73.3% for CIV infusion and significantly lower at 26.7% for IIV infusion [26]. In the same study, the time it took to reach therapeutic range was shorter (3.9 days for CIV infusion vs. 5.22 days for IIV infusion), and the incidence of renal toxicity was lower (23.3% for CIV infusion vs. 53.8% for IIV infusion) when vancomycin was infused continuously [26]. Another retrospective study performed on adult patients with ventilator-associated pneumonia caused by oxacillin-resistant S. aureus reported that vancomycin CIV administration was associated with a lower mortality rate of 25% compared to a mortality rate of 54.2% in patients who underwent treatment with the IIV infusion method or other antibiotics [27].
In addition, a high peak concentration of vancomycin is not required for antibiotic efficacy because vancomycin is not a concentration-dependent antibiotic [28]. In turn, it makes the trough vancomycin level and its measurement highly important, and the correct timing of a trough measurement becomes of utmost importance [28]. However, measuring a trough measurement within 30 min prior to the subsequent dose may not always be possible in a clinical setting, and inaccurate timing of sampling may result in a misleading vancomycin level. Since the serum concentration of vancomycin becomes linear after reaching a steady state with CIV administration, blood samples for TDM can be collected regardless of time, as long as the serum concentration has reached a steady state [28]. The adjustment of the vancomycin dosage can be done according to a more accurate serum concentration [28].
However, even with these benefits, vancomycin CIV infusion is not a protective method against low efficacy and nephrotoxicity. A study by Ingram et al. [29] reported that there was no significant difference in the risk of nephrotoxicity between CIV and IIV. A meta-analysis study carried out by Cataldo et al. [30] indeed concluded that vancomycin CIV infusion was associated with a lower risk of nephrotoxicity than IIV. Nevertheless, there was no difference in overall mortality between the two groups. Genuini et al. [12] conducted a retrospective study in which vancomycin was administered as a CIV infusion to pediatric patients who were admitted to the PICU. The study found that pediatric patients receiving vancomycin CIV infusions often showed subtherapeutic levels, which led to treatment failure [10]. Another minor difficulty associated with CIV infusion is that a dedicated intravenous route is necessary due to the incompatibility of vancomycin with other drugs.
This study has several limitations. First, this study excluded patients that were on CRRT or ECMO in an effort to eliminate factors that may have an excessive impact on renal clearance or renal blood flow, making our prediction model unsuitable for patients on CRRT or ECMO. However, one study reported that vancomycin serum concentration is more likely to be in the therapeutic range when vancomycin CIV infusion is used for patients on CRRT [31]. In another study, vancomycin CIV infusion for patients with CRRT was sufficiently advantageous over IIV infusion because severe nephrotoxicity or leukopenia were not observed in the CIV infusion group [32]. Also, patients in the CIV infusion group showed a generally higher serum concentration of vancomycin compared to the IIV infusion group [32]. Therefore, future studies need to be performed to identify equipment-related and hemodynamic factors that may affect renal clearance. It will enable us to derive a more robust and accurate prediction model of vancomycin serum concentration, even for patients on CRRT and ECMO. Secondly, this is a retrospective single-center study that enrolled only a small number of patients and TDM cases. Conducting a prospective, large-scale study that encompasses multiple centers and institutions is necessary for the validation of the predictive model. Lastly, as mentioned earlier, the coefficient of determination was relatively low at R2 = 0.66, and it would not be suitable to apply this equation in a clinical setting. It would be possible for us to modify and formulate an equation with more predictive power once more data has been accumulated.
In conclusion, we identified that the factors affecting vancomycin serum concentration during vancomycin CIV infusion are vancomycin dosage and serum creatinine. Then, we were able to derive an equation to calculate the vancomycin serum concentration. With the knowledge that renal clearance is an important factor in using vancomycin, careful titration of the drug based on renal clearance will allow for more effective and safe outcomes. Also, we believe that this prediction model will be able to contribute to establishing an effective vancomycin CIV dosing regimen for critically ill pediatric patients that vary in renal clearance.
REFERENCES
1. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Talan DA, Chambers HF. J Rybak M. 2011; Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 52:285–292. DOI: 10.1093/cid/cir034. PMID: 21217178.

2. Wysocki M, Delatour F, Faurisson F, Rauss A, Pean Y, Misset B, Thomas F, Timsit JF, Similowski T, Mentec H, Mier L, Dreyfuss D. 2001; Continuous versus intermittent infusion of vancomycin in severe Staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother. 45:2460–2467. DOI: 10.1128/AAC.45.9.2460-2467.2001. PMID: 11502515. PMCID: PMC90678.

3. Hao JJ, Chen H, Zhou JX. 2016; Continuous versus intermittent infusion of vancomycin in adult patients: a systematic review and meta-analysis. Int J Antimicrob Agents. 47:28–35. DOI: 10.1016/j.ijantimicag.2015.10.019. PMID: 26655032.

4. Hong LT, Goolsby TA, Sherman DS, Mueller SW, Reynolds P, Cava L, Neumann R, Kiser TH. 2015; Continuous infusion vs intermittent vancomycin in neurosurgical intensive care unit patients. J Crit Care. 30:1153.e1–6. DOI: 10.1016/j.jcrc.2015.06.012. PMID: 26239323.

5. Roberts JA, Taccone FS, Udy AA, Vincent JL, Jacobs F, Lipman J. 2011; Vancomycin dosing in critically ill patients: robust methods for improved continuous-infusion regimens. Antimicrob Agents Chemother. 55:2704–2709. DOI: 10.1128/AAC.01708-10. PMID: 21402850. PMCID: PMC3101407.

6. Cristallini S, Hites M, Kabtouri H, Roberts JA, Beumier M, Cotton F, Lipman J, Jacobs F, Vincent JL, Creteur J, Taccone FS. 2016; New regimen for continuous infusion of vancomycin in critically ill patients. Antimicrob Agents Chemother. 60:4750–4756. DOI: 10.1128/AAC.00330-16. PMID: 27216073. PMCID: PMC4958221.

7. da Silva Alves GC, da Silva SD, Frade VP, Rodrigues D, Baldoni AO, de Castro WV, Sanches C. 2017; Determining the optimal vancomycin daily dose for pediatrics: a meta-analysis. Eur J Clin Pharmacol. 73:1341–1353. DOI: 10.1007/s00228-017-2306-3. PMID: 28776198.

8. Udy AA, Baptista JP, Lim NL, Joynt GM, Jarrett P, Wockner L, Boots RJ, Lipman J. 2014; Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations*. Crit Care Med. 42:520–527. DOI: 10.1097/CCM.0000000000000029. PMID: 24201175.
9. Hurst AL, Baumgartner C, MacBrayne CE, Child J. 2019; Experience with continuous infusion vancomycin dosing in a large pediatric hospital. J Pediatric Infect Dis Soc. 8:174–179. DOI: 10.1093/jpids/piy032. PMID: 29718415.

10. McKamy S, Chen T, Lee M, Ambrose PJ. 2012; Evaluation of a pediatric continuous-infusion vancomycin therapy guideline. Am J Health Syst Pharm. 69:2066–2071. DOI: 10.2146/ajhp120072. PMID: 23172265.

11. Cies JJ, Moore WS 2nd, Conley SB, Muneeruddin S, Parker J, Shea P, Chopra A. 2016; Continuous infusion vancomycin through the addition of vancomycin to the continuous renal replacement therapy solution in the PICU: a case series. Pediatr Crit Care Med. 17:e138–e145. DOI: 10.1097/PCC.0000000000000656. PMID: 26890194.
12. Genuini M, Oualha M, Bouazza N, Moulin F, Treluyer JM, Lesage F, Renolleau S, Benaboud S. 2018; Achievement of therapeutic vancomycin exposure with continuous infusion in critically ill children. Pediatr Crit Care Med. 19:e263–e269. DOI: 10.1097/PCC.0000000000001474. PMID: 29394210.

13. Guilhaumou R, Marsot A, Dupouey J, Galambrun C, Boulamery A, Coze C, Simon N, André N. 2016; Pediatric patients with solid or hematological tumor disease: vancomycin population pharmacokinetics and dosage optimization. Ther Drug Monit. 38:559–566. DOI: 10.1097/FTD.0000000000000318. PMID: 27631462.

14. Hoegy D, Goutelle S, Garnier N, Rénard C, Faure-Conter C, Bergeron C, Bertrand Y, Bleyzac N. 2018; Continuous intravenous vancomycin in children with normal renal function hospitalized in hematology-oncology: prospective validation of a dosing regimen optimizing steady-state concentration. Fundam Clin Pharmacol. 32:323–329. DOI: 10.1111/fcp.12344. PMID: 29315849.

15. Plan O, Cambonie G, Barbotte E, Meyer P, Devine C, Milesi C, Pidoux O, Badr M, Picaud JC. 2008; Continuous-infusion vancomycin therapy for preterm neonates with suspected or documented Gram-positive infections: a new dosage schedule. Arch Dis Child Fetal Neonatal Ed. 93:F418–F421. Erratum in: Arch Dis Child Fetal Neonatal Ed. 2009;94:F78. DOI: 10.1136/adc.2007.128280. PMID: 18450803.

16. Patel AD, Anand D, Lucas C, Thomson AH. 2013; Continuous infusion of vancomycin in neonates. Arch Dis Child. 98:478–479. DOI: 10.1136/archdischild-2012-303197. PMID: 23543265.

17. de Hoog M, Mouton JW, van den Anker JN. 2004; Vancomycin: pharmacokinetics and administration regimens in neonates. Clin Pharmacokinet. 43:417–440. DOI: 10.2165/00003088-200443070-00001. PMID: 15139793.
18. Rainkie D, Ensom MH, Carr R. 2015; Pediatric assessment of vancomycin empiric dosing (PAVED): a retrospective review. Paediatr Drugs. 17:245–253. DOI: 10.1007/s40272-015-0122-8. PMID: 25813682.

19. Marsot A, Boulamery A, Bruguerolle B, Simon N. 2012; Vancomycin: a review of population pharmacokinetic analyses. Clin Pharmacokinet. 51:1–13. DOI: 10.2165/11596390-000000000-00000. PMID: 22149255.
20. Stewart JJ, Jorgensen SC, Dresser L, Lau TT, Gin A, Thirion DJ, Nishi C, Dalton B. 2021; A Canadian perspective on the revised 2020 ASHP-IDSA-PIDS-SIDP guidelines for vancomycin AUC-based therapeutic drug monitoring for serious MRSA infections. J Assoc Med Microbiol Infect Dis Can. 6:3–9. DOI: 10.3138/jammi-2020-0028. PMID: 36340210. PMCID: PMC9612435.

21. Mould DR, Upton RN. 2012; Basic concepts in population modeling, simulation, and model-based drug development. CPT Pharmacometrics Syst Pharmacol. 1:e6. DOI: 10.1038/psp.2012.4. PMID: 23835886. PMCID: PMC3606044.

22. Spapen HD, Janssen van Doorn K, Diltoer M, Verbrugghe W, Jacobs R, Dobbeleir N, Honoré PM, Jorens PG. 2011; Retrospective evaluation of possible renal toxicity associated with continuous infusion of vancomycin in critically ill patients. Ann Intensive Care. 1:26. DOI: 10.1186/2110-5820-1-26. PMID: 21906376. PMCID: PMC3224465.

23. Saugel B, Gramm C, Wagner JY, Messer M, Lahmer T, Meidert AS, Schmid RM, Huber W. 2014; Evaluation of a dosing regimen for continuous vancomycin infusion in critically ill patients: an observational study in intensive care unit patients. J Crit Care. 29:351–355. DOI: 10.1016/j.jcrc.2013.12.007. PMID: 24456810.

24. Pea F, Furlanut M, Negri C, Pavan F, Crapis M, Cristini F, Viale P. 2009; Prospectively validated dosing nomograms for maximizing the pharmacodynamics of vancomycin administered by continuous infusion in critically ill patients. Antimicrob Agents Chemother. 53:1863–1867. DOI: 10.1128/AAC.01149-08. PMID: 19223642. PMCID: PMC2681515.

25. Buyle FM, Decruyenaere J, De Waele J, Tulkens PM, Van Audenrode T, Depuydt P, Claeys G, Robays H, Vogelaers D. 2013; A survey of beta-lactam antibiotics and vancomycin dosing strategies in intensive care units and general wards in Belgian hospitals. Eur J Clin Microbiol Infect Dis. 32:763–768. DOI: 10.1007/s10096-012-1803-7. PMID: 23271675.

26. Schlobohm CJ, Zhu E, Duby JJ. 2021; Continuous infusion versus intermittent infusion vancomycin in a burn center intensive care unit. Burns. 47:1495–1501. DOI: 10.1016/j.burns.2021.08.016. PMID: 34538672.

27. Rello J, Sole-Violan J, Sa-Borges M, Garnacho-Montero J, Muñoz E, Sirgo G, Olona M, Diaz E. 2005; Pneumonia caused by oxacillin-resistant Staphylococcus aureus treated with glycopeptides. Crit Care Med. 33:1983–1987. DOI: 10.1097/01.CCM.0000178180.61305.1D. PMID: 16148469.

28. Waineo MF, Kuhn TC, Brown DL. 2015; The pharmacokinetic/pharmacodynamic rationale for administering vancomycin via continuous infusion. J Clin Pharm Ther. 40:259–265. DOI: 10.1111/jcpt.12270. PMID: 25865426.

29. Ingram PR, Lye DC, Tambyah PA, Goh WP, Tam VH, Fisher DA. 2008; Risk factors for nephrotoxicity associated with continuous vancomycin infusion in outpatient parenteral antibiotic therapy. J Antimicrob Chemother. 62:168–171. DOI: 10.1093/jac/dkn080. PMID: 18334494.
30. Cataldo MA, Tacconelli E, Grilli E, Pea F, Petrosillo N. 2012; Continuous versus intermittent infusion of vancomycin for the treatment of Gram-positive infections: systematic review and meta-analysis. J Antimicrob Chemother. 67:17–24. DOI: 10.1093/jac/dkr442. PMID: 22028203.

31. Akers KS, Cota JM, Chung KK, Renz EM, Mende K, Murray CK. 2012; Serum vancomycin levels resulting from continuous or intermittent infusion in critically ill burn patients with or without continuous renal replacement therapy. J Burn Care Res. 33:e254–e262. DOI: 10.1097/BCR.0b013e31825042fa. PMID: 22878490.

32. Covajes C, Scolletta S, Penaccini L, Ocampos-Martinez E, Abdelhadii A, Beumier M, Jacobs F, de Backer D, Vincent JL, Taccone FS. 2013; Continuous infusion of vancomycin in septic patients receiving continuous renal replacement therapy. Int J Antimicrob Agents. 41:261–266. DOI: 10.1016/j.ijantimicag.2012.10.018. PMID: 23312601.

Fig. 1
Patient selection.
IIV, intermittent intravenous; CIV, continuous intravenous; SCr, serum creatinine; TDM, therapeutic drug monitoring; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation.
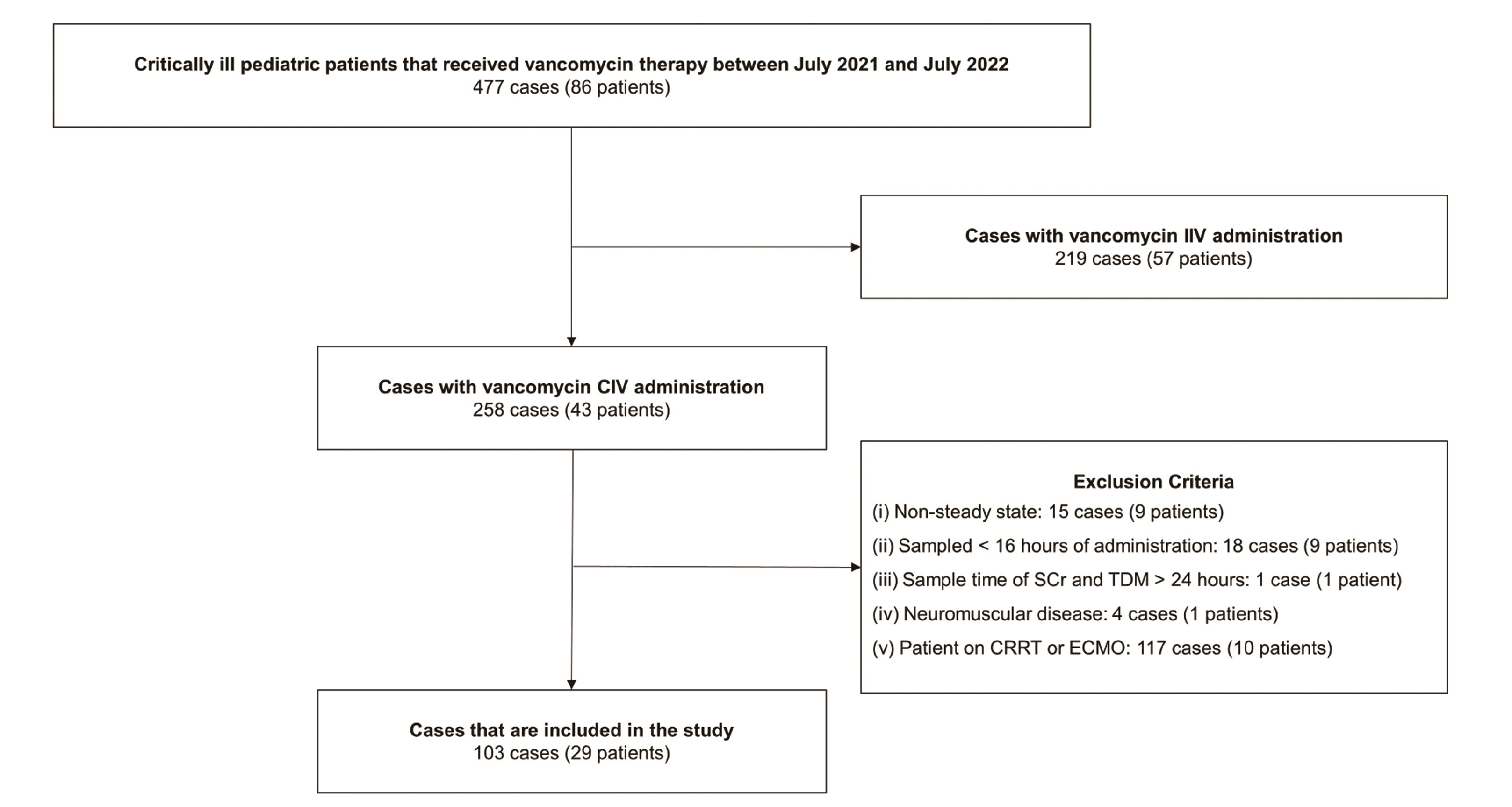
Fig. 2
Plot of observed vancomycin serum concentration
vs. model-predicted vancomycin serum concentration. The median value of the predicted vancomycin serum concentrations for the study population was 21.2 (17.1–25.1) mg/l, comparable to the observed vancomycin serum concentration of 22.1 (18.3–24.6) mg/l. IQR, interquartile range.
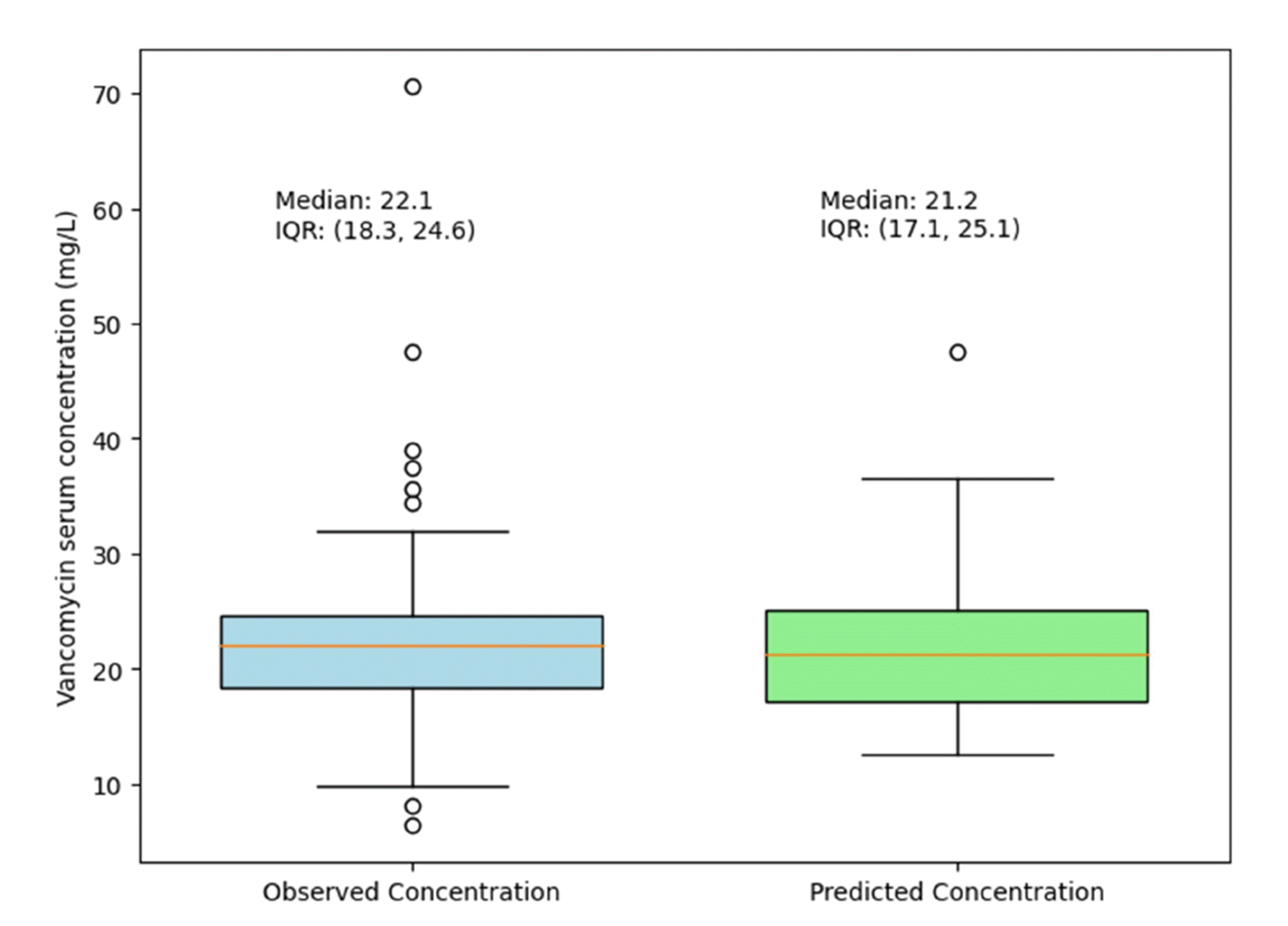
Table 1
Baseline characteristics of the cases
Continuous data are presented as medians (interquartile range), and categorical data are presented as numbers (%). aNot applicable because the values vary depending on the timing of samples. bThe daily dosing was adjusted according to the therapeutic drug monitoring report. cTime between vancomycin administration and therapeutic drug monitoring sample collection.
Table 2
Factors affecting vancomycin concentration




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download