This article has been
cited by other articles in ScienceCentral.
Abstract
Guillain–Barré syndrome (GBS) is an autoimmune-driven condition characterized by acute polyneuropathy, often emerging as a sequel to prior infections or vaccinations. This study presents the first reported cases of GBS emerging after the full recovery from coronavirus disease 2019 (COVID-19) infection in Korea. Despite experiencing mild acute COVID-19 symptoms, these patients faced substantial weakness attributed to GBS, significantly affecting their daily lives. The timely administration of intravenous immunoglobulin treatment halted the progression of symptoms, underscoring the critical importance of early intervention. These cases highlight the potential for neurological complications associated with COVID-19 and underscore the necessity for continuous monitoring and timely medical care.
Keywords: Guillain–Barré Syndrome, GBS, COVID-19, SARS-CoV-2, Peripheral Nervous System
INTRODUCTION
The rapid global spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), giving rise to the novel coronavirus disease 2019 (COVID-19), has led to an ongoing pandemic. While the clinical spectrum of COVID-19 encompasses mild respiratory symptoms to severe respiratory distress, multi-organ failure, and even mortality, emerging evidence now underscores the possibility of diverse neurological complications associated with this viral infection.
1
Amidst the primary attention devoted to respiratory manifestations, it is becoming increasingly apparent that COVID-19’s impact may extend beyond the respiratory system. One noteworthy neurological complication is Guillain–Barré syndrome (GBS), a relatively uncommon yet severe autoimmune disorder targeting the peripheral nervous system.
2 GBS manifests through acute muscle weakness, often accompanied by sensory anomalies and potential paralysis. Its etiology involves an immune response gone awry, often incited by infections, which can result in the damage of peripheral nerves through demyelination or axonal disruption. Throughout medical literature, a link between COVID-19 infection and GBS emergence has been observed.
34567
Through a series of cases illustrating the emergence of GBS following the full recovery from COVID-19 infection in Korea, our aim is to enrich the comprehension of the neurological consequences linked to COVID-19, placing special emphasis on these being the initial reported cases within the country.
CASE DESCRIPTION
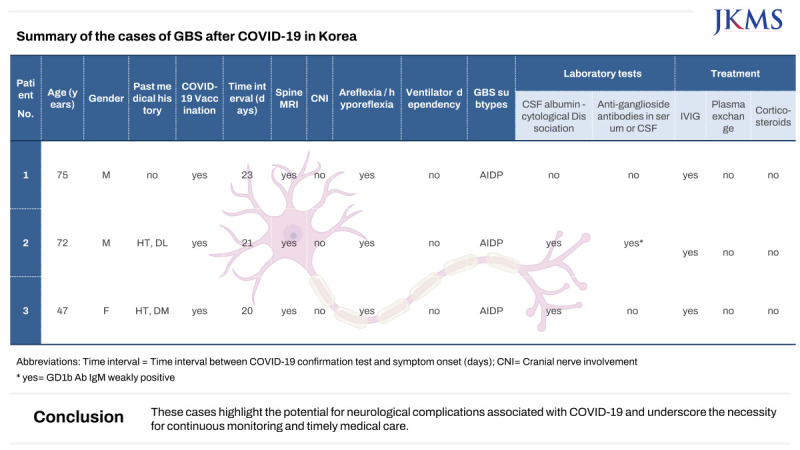
Case 1
A 75-year-old man, previously healthy, received the first and second doses of the AstraZeneca COVID-19 vaccine on May 31, 2021, and August 16, 2021, respectively. He also received the third dose of the Pfizer vaccine on December 13, 2021. There were no significant adverse effects observed following the vaccinations.
On February 18, 2022, the patient presented with symptoms of cough and sore throat, and tested positive for SARS-CoV-2 through a polymerase chain reaction (PCR) test. Subsequently, the patient underwent a 7-day period of self-isolation at home, as the cold-like symptoms were not severe, and acetaminophen was administered. Twenty-three days after the confirmation of COVID-19, on March 13, 2022, the patient experienced bilateral symmetrical weakness in the lower extremities. The symptoms gradually progressed, with a tingling sensation ascending from the feet. On March 22, 2022, the patient was admitted to an orthopedic specialized hospital, where a spinal magnetic resonance imaging (MRI) was performed, revealing no significant abnormalities. As the symptoms progressed, the patient was transferred to our institution for further evaluation. At the time of the patient's hospital admission, the motor power of both lower limbs was assessed as Medical Research Council (MRC) grade III, with distal power slightly diminished to grade II. Additionally, the patient exhibited paresthesia in both upper and lower limbs. Deep tendon reflexes (DTRs) were absent in both lower limbs, while there were no signs of facial or upper limb muscle weakness. Routine laboratory tests, GBS antibodies, and other virus antibodies including
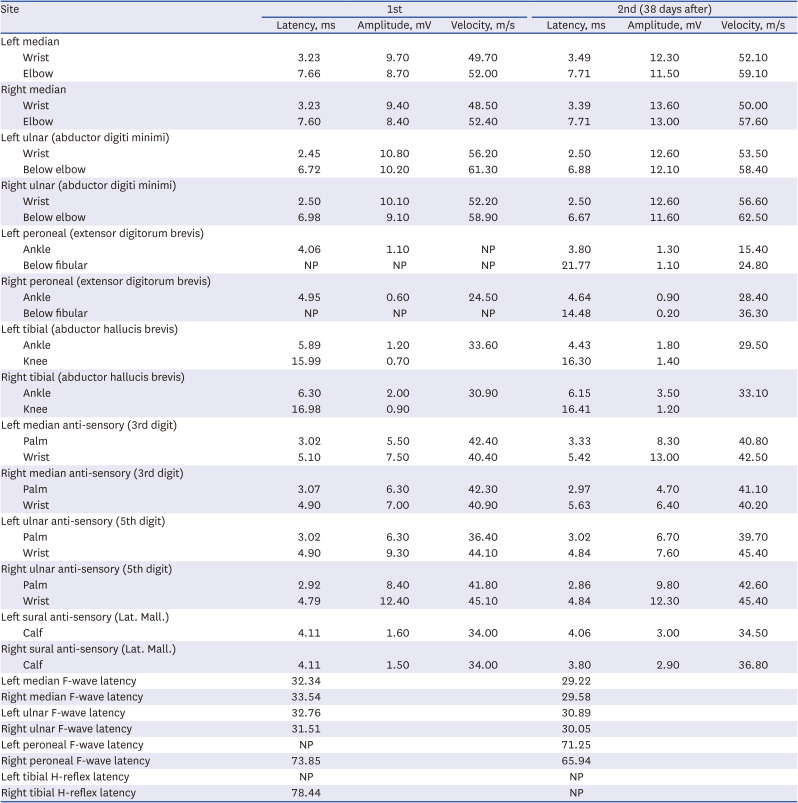
Mycoplasma pneumoniae, Epstein-Barr virus, cytomegalovirus were conducted, resulting in no abnormalities. Cerebrospinal fluid analysis showed no albumin-cytologic dissociation and any sign of infection (no white blood & red blood cells and protein 44.0 mg/dL). Nerve conduction study results showed a demyelinating-type polyneuropathy (
Table 1). The patient received intravenous immunoglobulin (IVIG) therapy for 5 days. The patient reported that the progression of symptoms seemed to halt from the second day of IVIG administration, and from the third day onward, he noticed a slightly increased strength in both toes. During his hospitalization, there was a gradual return of motor power in the lower limbs, although he remained dependent on a wheelchair upon discharge. Thirty-eight days later, during an outpatient visit, he underwent follow-up nerve conduction study (NCS) and a neurologic examination. The motor power of both lower limbs had improved to approximately grade IV, yet DTRs in the lower limbs were still absent.
Table 1
Nerve conduction study and subsequent follow-up in case 1
|
Site |
1st |
2nd (38 days after) |
|
Latency, ms |
Amplitude, mV |
Velocity, m/s |
Latency, ms |
Amplitude, mV |
Velocity, m/s |
|
Left median |
|
|
|
|
|
|
|
Wrist |
3.23 |
9.70 |
49.70 |
3.49 |
12.30 |
52.10 |
|
Elbow |
7.66 |
8.70 |
52.00 |
7.71 |
11.50 |
59.10 |
|
Right median |
|
|
|
|
|
|
|
Wrist |
3.23 |
9.40 |
48.50 |
3.39 |
13.60 |
50.00 |
|
Elbow |
7.60 |
8.40 |
52.40 |
7.71 |
13.00 |
57.60 |
|
Left ulnar (abductor digiti minimi) |
|
|
|
|
|
|
|
Wrist |
2.45 |
10.80 |
56.20 |
2.50 |
12.60 |
53.50 |
|
Below elbow |
6.72 |
10.20 |
61.30 |
6.88 |
12.10 |
58.40 |
|
Right ulnar (abductor digiti minimi) |
|
|
|
|
|
|
|
Wrist |
2.50 |
10.10 |
52.20 |
2.50 |
12.60 |
56.60 |
|
Below elbow |
6.98 |
9.10 |
58.90 |
6.67 |
11.60 |
62.50 |
|
Left peroneal (extensor digitorum brevis) |
|
|
|
|
|
|
|
Ankle |
4.06 |
1.10 |
NP |
3.80 |
1.30 |
15.40 |
|
Below fibular |
NP |
NP |
NP |
21.77 |
1.10 |
24.80 |
|
Right peroneal (extensor digitorum brevis) |
|
|
|
|
|
|
|
Ankle |
4.95 |
0.60 |
24.50 |
4.64 |
0.90 |
28.40 |
|
Below fibular |
NP |
NP |
NP |
14.48 |
0.20 |
36.30 |
|
Left tibial (abductor hallucis brevis) |
|
|
|
|
|
|
|
Ankle |
5.89 |
1.20 |
33.60 |
4.43 |
1.80 |
29.50 |
|
Knee |
15.99 |
0.70 |
|
16.30 |
1.40 |
|
|
Right tibial (abductor hallucis brevis) |
|
|
|
|
|
|
|
Ankle |
6.30 |
2.00 |
30.90 |
6.15 |
3.50 |
33.10 |
|
Knee |
16.98 |
0.90 |
|
16.41 |
1.20 |
|
|
Left median anti-sensory (3rd digit) |
|
|
|
|
|
|
|
Palm |
3.02 |
5.50 |
42.40 |
3.33 |
8.30 |
40.80 |
|
Wrist |
5.10 |
7.50 |
40.40 |
5.42 |
13.00 |
42.50 |
|
Right median anti-sensory (3rd digit) |
|
|
|
|
|
|
|
Palm |
3.07 |
6.30 |
42.30 |
2.97 |
4.70 |
41.10 |
|
Wrist |
4.90 |
7.00 |
40.90 |
5.63 |
6.40 |
40.20 |
|
Left ulnar anti-sensory (5th digit) |
|
|
|
|
|
|
|
Palm |
3.02 |
6.30 |
36.40 |
3.02 |
6.70 |
39.70 |
|
Wrist |
4.90 |
9.30 |
44.10 |
4.84 |
7.60 |
45.40 |
|
Right ulnar anti-sensory (5th digit) |
|
|
|
|
|
|
|
Palm |
2.92 |
8.40 |
41.80 |
2.86 |
9.80 |
42.60 |
|
Wrist |
4.79 |
12.40 |
45.10 |
4.84 |
12.30 |
45.40 |
|
Left sural anti-sensory (Lat. Mall.) |
|
|
|
|
|
|
|
Calf |
4.11 |
1.60 |
34.00 |
4.06 |
3.00 |
34.50 |
|
Right sural anti-sensory (Lat. Mall.) |
|
|
|
|
|
|
|
Calf |
4.11 |
1.50 |
34.00 |
3.80 |
2.90 |
36.80 |
|
Left median F-wave latency |
32.34 |
|
|
29.22 |
|
|
|
Right median F-wave latency |
33.54 |
|
|
29.58 |
|
|
|
Left ulnar F-wave latency |
32.76 |
|
|
30.89 |
|
|
|
Right ulnar F-wave latency |
31.51 |
|
|
30.05 |
|
|
|
Left peroneal F-wave latency |
NP |
|
|
71.25 |
|
|
|
Right peroneal F-wave latency |
73.85 |
|
|
65.94 |
|
|
|
Left tibial H-reflex latency |
NP |
|
|
NP |
|
|
|
Right tibial H-reflex latency |
78.44 |
|
|
NP |
|
|
Case 2
A 72-year-old man with a history of well-controlled hypertension and hyperlipidemia had received Pfizer vaccines for COVID-19 prevention. Although he couldn't recall the exact dates of the first and second doses, he received the final third dose on December 13, 2021, with no reported adverse reactions.
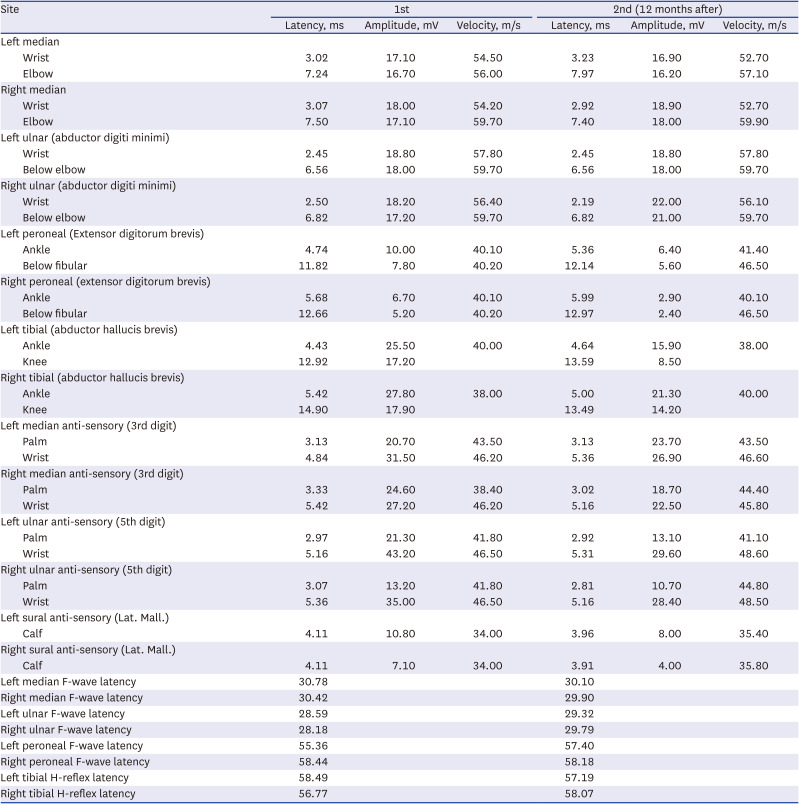
On March 13, 2022, the patient tested positive for COVID-19 in a PCR nasal swab. At that time, he experienced mild body aches and took nonsteroidal anti-inflammatory drugs. On April 3, 2022, which was 21 days after the COVID-19 confirmation, he began experiencing numbness in both feet. Seven days later, he developed bilateral symmetrical weakness in his lower extremities, which gradually worsened in an ascending pattern. At the time of admission, the proximal part of both lower limbs was assessed as MRC grade IV, and the distal part as grade II, leading him to be wheelchair-bound. He reported reduced sensation to light touch and pain in the lower extremities, and paresthesia was observed in both upper and lower limbs. DTRs were hypoactive. Basic blood tests, brain MRI, and other assessments did not reveal any notable abnormalities, except for a weakly positive result in the GD1b Ab IgM test. Cerebrospinal fluid analysis indicated albumin-cytologic dissociation without signs of infection (3 white blood cells/μL, 0 red blood cells/μL, and protein 53.3 mg/dL). NCS revealed demyelinating polyneuropathy with abnormal F-waves (
Table 2). The patient received a 5-day course of IVIG treatment. From the second day of IVIG administration, he reported that his symptoms no longer worsened. His leg strength improved to MRC grade III, but independent walking remains challenging. A follow-up was conducted 8 months after discharge, and NCS was performed as part of follow-up. Although improvements were observed in motor and sensory abnormalities, he still required the use of a walker for mobility, and hyporeflexia was observed in DTRs.
Table 2
Nerve conduction study and subsequent follow-up in case 2
|
Site |
1st |
2nd (12 months after) |
|
Latency, ms |
Amplitude, mV |
Velocity, m/s |
Latency, ms |
Amplitude, mV |
Velocity, m/s |
|
Left median |
|
|
|
|
|
|
|
Wrist |
3.02 |
17.10 |
54.50 |
3.23 |
16.90 |
52.70 |
|
Elbow |
7.24 |
16.70 |
56.00 |
7.97 |
16.20 |
57.10 |
|
Right median |
|
|
|
|
|
|
|
Wrist |
3.07 |
18.00 |
54.20 |
2.92 |
18.90 |
52.70 |
|
Elbow |
7.50 |
17.10 |
59.70 |
7.40 |
18.00 |
59.90 |
|
Left ulnar (abductor digiti minimi) |
|
|
|
|
|
|
|
Wrist |
2.45 |
18.80 |
57.80 |
2.45 |
18.80 |
57.80 |
|
Below elbow |
6.56 |
18.00 |
59.70 |
6.56 |
18.00 |
59.70 |
|
Right ulnar (abductor digiti minimi) |
|
|
|
|
|
|
|
Wrist |
2.50 |
18.20 |
56.40 |
2.19 |
22.00 |
56.10 |
|
Below elbow |
6.82 |
17.20 |
59.70 |
6.82 |
21.00 |
59.70 |
|
Left peroneal (Extensor digitorum brevis) |
|
|
|
|
|
|
|
Ankle |
4.74 |
10.00 |
40.10 |
5.36 |
6.40 |
41.40 |
|
Below fibular |
11.82 |
7.80 |
40.20 |
12.14 |
5.60 |
46.50 |
|
Right peroneal (extensor digitorum brevis) |
|
|
|
|
|
|
|
Ankle |
5.68 |
6.70 |
40.10 |
5.99 |
2.90 |
40.10 |
|
Below fibular |
12.66 |
5.20 |
40.20 |
12.97 |
2.40 |
46.50 |
|
Left tibial (abductor hallucis brevis) |
|
|
|
|
|
|
|
Ankle |
4.43 |
25.50 |
40.00 |
4.64 |
15.90 |
38.00 |
|
Knee |
12.92 |
17.20 |
|
13.59 |
8.50 |
|
|
Right tibial (abductor hallucis brevis) |
|
|
|
|
|
|
|
Ankle |
5.42 |
27.80 |
38.00 |
5.00 |
21.30 |
40.00 |
|
Knee |
14.90 |
17.90 |
|
13.49 |
14.20 |
|
|
Left median anti-sensory (3rd digit) |
|
|
|
|
|
|
|
Palm |
3.13 |
20.70 |
43.50 |
3.13 |
23.70 |
43.50 |
|
Wrist |
4.84 |
31.50 |
46.20 |
5.36 |
26.90 |
46.60 |
|
Right median anti-sensory (3rd digit) |
|
|
|
|
|
|
|
Palm |
3.33 |
24.60 |
38.40 |
3.02 |
18.70 |
44.40 |
|
Wrist |
5.42 |
27.20 |
46.20 |
5.16 |
22.50 |
45.80 |
|
Left ulnar anti-sensory (5th digit) |
|
|
|
|
|
|
|
Palm |
2.97 |
21.30 |
41.80 |
2.92 |
13.10 |
41.10 |
|
Wrist |
5.16 |
43.20 |
46.50 |
5.31 |
29.60 |
48.60 |
|
Right ulnar anti-sensory (5th digit) |
|
|
|
|
|
|
|
Palm |
3.07 |
13.20 |
41.80 |
2.81 |
10.70 |
44.80 |
|
Wrist |
5.36 |
35.00 |
46.50 |
5.16 |
28.40 |
48.50 |
|
Left sural anti-sensory (Lat. Mall.) |
|
|
|
|
|
|
|
Calf |
4.11 |
10.80 |
34.00 |
3.96 |
8.00 |
35.40 |
|
Right sural anti-sensory (Lat. Mall.) |
|
|
|
|
|
|
|
Calf |
4.11 |
7.10 |
34.00 |
3.91 |
4.00 |
35.80 |
|
Left median F-wave latency |
30.78 |
|
|
30.10 |
|
|
|
Right median F-wave latency |
30.42 |
|
|
29.90 |
|
|
|
Left ulnar F-wave latency |
28.59 |
|
|
29.32 |
|
|
|
Right ulnar F-wave latency |
28.18 |
|
|
29.79 |
|
|
|
Left peroneal F-wave latency |
55.36 |
|
|
57.40 |
|
|
|
Right peroneal F-wave latency |
58.44 |
|
|
58.18 |
|
|
|
Left tibial H-reflex latency |
58.49 |
|
|
57.19 |
|
|
|
Right tibial H-reflex latency |
56.77 |
|
|
58.07 |
|
|
Case 3
A 47-year-old female patient with a history of hypertension and diabetes received the Pfizer COVID-19 vaccine for the first time on September 6, 2021, and for the second time on October 21, 2021. After the second dose, she experienced a 5-month cessation of menstruation and did not receive any further vaccine doses.
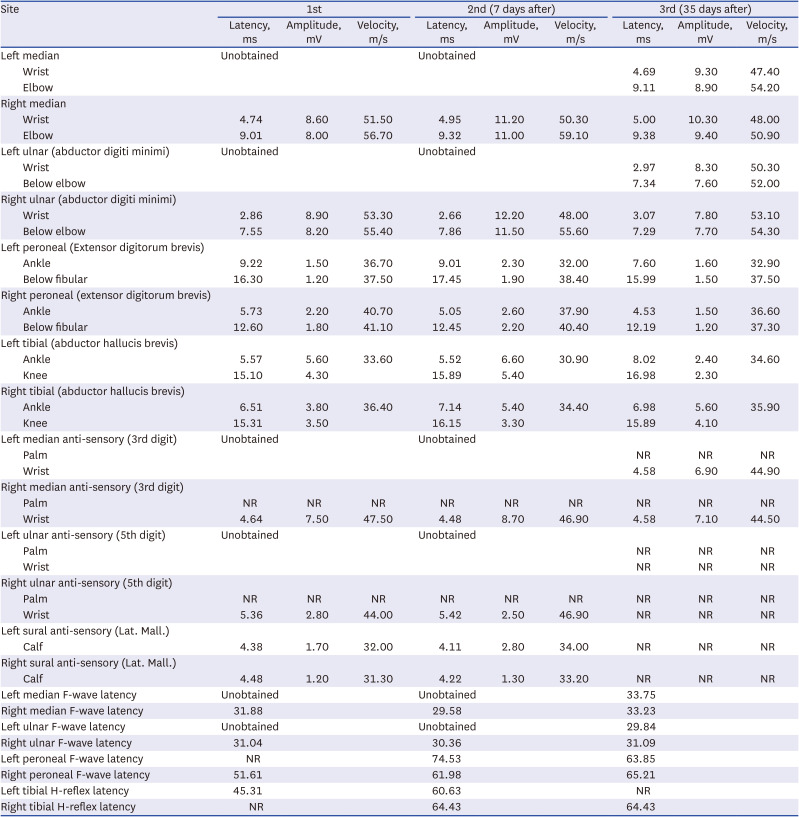
On August 4, 2022, she tested positive for COVID-19 through PCR test and received medical treatment for pneumonia symptoms, resulting in improvement. On August 28, 2022, she began experiencing numbness in both lower limbs, which progressed in an ascending pattern. This led to her admission to the hospital’s neurosurgery department where brain and spinal MRI were conducted. However, no significant abnormalities were found to explain her symptoms. Her motor power was assessed as MRC grade II in the proximal part and grade I in the distal part of both lower limbs. She reported severe paresthesia in the upper extremities and lower extremities, and DTRs were absent in both lower limbs. Basic blood tests revealed a glucose level of 362, and her hemoglobin A1C was 12.7%, indicating poorly controlled diabetes. GBS antibody tests did not show any specific findings. Cerebrospinal fluid analysis indicated albumin-cytologic dissociation without signs of infection (3 white blood cells/μL, 0 red blood cells/μL, and protein 226.9 mg/dL). NCS revealed a sensory-dominant mixed polyneuropathy with abnormal F-waves (
Table 3). She received IVIG therapy, and on the second day of IVIG administration, she reported a cessation in the progression of symptoms. Starting from the 4th day, she demonstrated improved dorsiflexion movement. Follow-up NCS were performed on the 7th and 35th days, revealing ongoing sensory symptoms and gradual motor improvement.
Table 3
Nerve conduction study and subsequent follow-up in case 3
|
Site |
1st |
2nd (7 days after) |
3rd (35 days after) |
|
Latency, ms |
Amplitude, mV |
Velocity, m/s |
Latency, ms |
Amplitude, mV |
Velocity, m/s |
Latency, ms |
Amplitude, mV |
Velocity, m/s |
|
Left median |
Unobtained |
|
|
Unobtained |
|
|
|
|
|
|
Wrist |
|
|
|
|
|
|
4.69 |
9.30 |
47.40 |
|
Elbow |
|
|
|
|
|
|
9.11 |
8.90 |
54.20 |
|
Right median |
|
|
|
|
|
|
|
|
|
|
Wrist |
4.74 |
8.60 |
51.50 |
4.95 |
11.20 |
50.30 |
5.00 |
10.30 |
48.00 |
|
Elbow |
9.01 |
8.00 |
56.70 |
9.32 |
11.00 |
59.10 |
9.38 |
9.40 |
50.90 |
|
Left ulnar (abductor digiti minimi) |
Unobtained |
|
|
Unobtained |
|
|
|
|
|
|
Wrist |
|
|
|
|
|
|
2.97 |
8.30 |
50.30 |
|
Below elbow |
|
|
|
|
|
|
7.34 |
7.60 |
52.00 |
|
Right ulnar (abductor digiti minimi) |
|
|
|
|
|
|
|
|
|
|
Wrist |
2.86 |
8.90 |
53.30 |
2.66 |
12.20 |
48.00 |
3.07 |
7.80 |
53.10 |
|
Below elbow |
7.55 |
8.20 |
55.40 |
7.86 |
11.50 |
55.60 |
7.29 |
7.70 |
54.30 |
|
Left peroneal (Extensor digitorum brevis) |
|
|
|
|
|
|
|
|
|
|
Ankle |
9.22 |
1.50 |
36.70 |
9.01 |
2.30 |
32.00 |
7.60 |
1.60 |
32.90 |
|
Below fibular |
16.30 |
1.20 |
37.50 |
17.45 |
1.90 |
38.40 |
15.99 |
1.50 |
37.50 |
|
Right peroneal (extensor digitorum brevis) |
|
|
|
|
|
|
|
|
|
|
Ankle |
5.73 |
2.20 |
40.70 |
5.05 |
2.60 |
37.90 |
4.53 |
1.50 |
36.60 |
|
Below fibular |
12.60 |
1.80 |
41.10 |
12.45 |
2.20 |
40.40 |
12.19 |
1.20 |
37.30 |
|
Left tibial (abductor hallucis brevis) |
|
|
|
|
|
|
|
|
|
|
Ankle |
5.57 |
5.60 |
33.60 |
5.52 |
6.60 |
30.90 |
8.02 |
2.40 |
34.60 |
|
Knee |
15.10 |
4.30 |
|
15.89 |
5.40 |
|
16.98 |
2.30 |
|
|
Right tibial (abductor hallucis brevis) |
|
|
|
|
|
|
|
|
|
|
Ankle |
6.51 |
3.80 |
36.40 |
7.14 |
5.40 |
34.40 |
6.98 |
5.60 |
35.90 |
|
Knee |
15.31 |
3.50 |
|
16.15 |
3.30 |
|
15.89 |
4.10 |
|
|
Left median anti-sensory (3rd digit) |
Unobtained |
|
|
Unobtained |
|
|
|
|
|
|
Palm |
|
|
|
|
|
|
NR |
NR |
NR |
|
Wrist |
|
|
|
|
|
|
4.58 |
6.90 |
44.90 |
|
Right median anti-sensory (3rd digit) |
|
|
|
|
|
|
|
|
|
|
Palm |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
|
Wrist |
4.64 |
7.50 |
47.50 |
4.48 |
8.70 |
46.90 |
4.58 |
7.10 |
44.50 |
|
Left ulnar anti-sensory (5th digit) |
Unobtained |
|
|
Unobtained |
|
|
|
|
|
|
Palm |
|
|
|
|
|
|
NR |
NR |
NR |
|
Wrist |
|
|
|
|
|
|
NR |
NR |
NR |
|
Right ulnar anti-sensory (5th digit) |
|
|
|
|
|
|
|
|
|
|
Palm |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
NR |
|
Wrist |
5.36 |
2.80 |
44.00 |
5.42 |
2.50 |
46.90 |
NR |
NR |
NR |
|
Left sural anti-sensory (Lat. Mall.) |
|
|
|
|
|
|
|
|
|
|
Calf |
4.38 |
1.70 |
32.00 |
4.11 |
2.80 |
34.00 |
NR |
NR |
NR |
|
Right sural anti-sensory (Lat. Mall.) |
|
|
|
|
|
|
|
|
|
|
Calf |
4.48 |
1.20 |
31.30 |
4.22 |
1.30 |
33.20 |
NR |
NR |
NR |
|
Left median F-wave latency |
Unobtained |
|
|
Unobtained |
|
|
33.75 |
|
|
|
Right median F-wave latency |
31.88 |
|
|
29.58 |
|
|
33.23 |
|
|
|
Left ulnar F-wave latency |
Unobtained |
|
|
Unobtained |
|
|
29.84 |
|
|
|
Right ulnar F-wave latency |
31.04 |
|
|
30.36 |
|
|
31.09 |
|
|
|
Left peroneal F-wave latency |
NR |
|
|
74.53 |
|
|
63.85 |
|
|
|
Right peroneal F-wave latency |
51.61 |
|
|
61.98 |
|
|
65.21 |
|
|
|
Left tibial H-reflex latency |
45.31 |
|
|
60.63 |
|
|
NR |
|
|
|
Right tibial H-reflex latency |
NR |
|
|
64.43 |
|
|
64.43 |
|
|
DISCUSSION
Since the first confirmed case of COVID-19 in Korea after arrival from Wuhan in 2023,
8 COVID-19 infections have continued to occur. According to meta-analysis, as of October 2022, there have been approximately 436 reported cases of GBS following COVID-19 infection worldwide.
9 The average age was in the 60s, with a higher proportion of male patients. The most common symptom was weakness, and the most prevalent sign was hyporeflexia/areflexia in DTRs. The demyelinating variant of GBS (also called acute inflammatory demyelinating polyradiculoneuropathy; AIDP) was the most common subtype observed in NCS. While the exact pathophysiology is not yet clear, it is speculated that both the direct pathway of the SARS-CoV-2 virus and the indirect pathway through immune and inflammatory reactions might contribute to neurological complications.
10 Previous studies emphasized that the neurological impacts of COVID-19 are connected to inflammatory mechanisms, particularly the emergence of a cytokine storm.
1112 In our cases, both nasal swab and cerebrospinal fluid SARS-CoV-2 real-time PCR tests yielded negative results during the occurrence of lower limb weakness. This finding supports the hypothesis of immune-mediated nerve damage through the indirect pathway.
All patients in this study presented an acute ascending pattern of motor and sensory deficits, rendering them unable to walk independently. They exhibited reduced/absent DTRs and showed the demyelinating pattern on NCS. None of them had any motor or neurological symptoms prior to the onset of their symptoms, and there was no history or symptoms suggestive of preceding gastrointestinal or other infections. Furthermore, blood tests conducted upon admission did not show leukocytosis, and tests for other viral infections. At admission, none of the patients had acute respiratory symptoms, and chest X-rays were normal. IVIG therapy (400 mg/kg/day) was administered for 5 days once GBS was suspected. All three patients exhibited a cessation of symptom progression within 1–2 days of IVIG treatment, and none of them necessitated respiratory support or intensive care unit care. Nonetheless, a common thread emerges in the weakness resulting from GBS, significantly impacting their daily lives. During a longitudinal observation period of almost a year for two patients, challenges in walking due to persistent gait impairment endured.
A previous case related to GBS in the context of COVID-19 infection had been reported in Korea.
13 However, that instance was para-infectious GBS and our study represents the first case series in the country reporting GBS as a long-term complication emerging after the complete recovery of patients who experienced a mild course of COVID-19 without any lingering sequelae. To prevent potential severe complications resulting from delayed diagnosis and treatment of GBS following COVID-19 infection, it is crucial to maintain close monitoring for the long COVID-19 syndrome.
Ethics statement
This study was approved by the Institutional Review Board of Eunpyeong St. Mary’s Hospital and the requirement for informed consent was waived (IRB PC23ZISI0147).