INTRODUCTION
Human adenovirus (HAdV) is often associated with acute respiratory disease (ARD), ranging from mild infection to severe pneumonia. ARD represents a significant concern in the military setting because the military population is particularly vulnerable due to crowded living conditions, high-level training, and high-level stress. In the U.S. military, HAdV was the most common causative agent of respiratory tract infection before HAdV vaccines became available in the 1960s. Before the availability of HAdV vaccines, military recruits during training had a high likelihood of contracting the virus. Indeed, it was observed that up to 80% of recruits became infected with HAdV, and nearly 40% of those infected experienced significant illness, leading to a hospitalization rate of 20%.
1 However, the introduction of HAdV vaccines has significantly reduced the number of infection cases.
2
Even though coronavirus disease 2019 (COVID-19) has temporarily reduced the number of HAdV infection cases due to the implementation of various preventive measures, such as social distancing, mask use, and hand hygiene, HAdV-associated ARD was a public health concern in the South Korean military prior to the COVID-19 pandemic. Specifically, in the Korean military, HAdV-55 is repeatedly identified as the main subtype responsible for outbreaks.
34 Following the identification of fatal pneumonia cases associated with HAdV-55 in 2012, the first large HAdV-55 outbreak was reported in the winter of 2014, and since then, HAdV outbreaks have been reported continuously and sporadically.
5 A study conducted from 2013 to 2018 in the Korean military also revealed an ongoing, substantial outbreak of HAdV-associated ARD with a significant deviation from the typical seasonal patterns.
6 Moreover, in the Korean military, ARD caused by HAdV has poorer clinical outcomes compared to other respiratory viruses. According to a study of patients with febrile respiratory disease or pneumonia in all Korean military hospitals from October 2014 to May 2016, HAdV patients were at greater risk of being admitted to intensive care or receiving mechanical ventilation (MV) treatment than non-HAdV patients.
7 Another previous study, which observed and compared HAdV pneumonia patients with non-HAdV pneumonia patients in the Korean military between 2012 and 2016, discovered that HAdV-associated pneumonia had a higher incidence of symptoms such as fever, cough, sore throat, muscle pain, diarrhea, and shortness of breath, as well as a higher Pneumonia Severity Index.
3
To establish an appropriate intervention strategy in response to the HAdV outbreak within the Korean military, it is necessary to understand the current situation. Although a few previous studies have investigated HAdV outbreaks in the Korean military, they focused on narrow time frames or single-site analyses. Therefore, we aim to provide a more comprehensive epidemiological analysis of all confirmed cases of ARD within the entire military hospital over an extended period. We not only examined the prevalence of ARDs attributable to HAdV but also their medical utilization, treatment modalities, and prognosis. These were then compared with cases infected with non-HAdV. Moreover, we attempted to evaluate the follow-up care provided in civilian hospitals for patients who were transferred out.
METHODS
In Korean military, the New Defense Medical Information System (NDEMIS) collects and manages all medical-related information generated in all 14 Korean military hospitals, including electronic medical records, preventive duty activities, and various health statistics. The collected data are then integrated with the Defense Medical Statistical Information System (DMSIS) to obtain demographic variables, such as sex, military rank, and age, as well as clinical parameters, such as diagnosis, while ensuring the exclusion of personal identifiable information. From NDEMIS and DMSIS, we extracted information on patients who underwent respiratory virus polymerase chain reaction (RV-PCR) testing and tested positive from January 2013 to July 2022. In 2009, following the H1N1 pandemic, RV-PCR was introduced to Korean military hospitals, but it was only selectively used at that time; however, in response to the 2014–2015 HAdV-55 outbreak, RV-PCR was routinely performed on all patients with ARD. Furthermore, information regarding individuals who were transferred to civilian hospitals was obtained through a review of data from the Ministry of National Defense, Army Headquarters, Navy Headquarters, Air Force Headquarters, and Armed Forces Medical Command.
We retrospectively reviewed the study participants’ medical records for 3 months after the RV-PCR test date and collected the following information: 1) demographic information, including sex, age, military type, and rank; 2) test dates and results of RV-PCR; 3) dates and corresponding diagnosis of emergency room, outpatient, inpatient, and intensive care unit (ICU) visits; 4) transfer or death records with corresponding diagnosis; and 5) treatment received.
Supplementary Table 1 illustrates the four types of RV-PCR tests used during the study period. The following respiratory viruses are detectable by the PCR test used in Korean military hospitals: HAdV, human bocavirus (HBoV), human coronavirus (HCoV), human enterovirus (HEV), influenza virus (IFV), human metapneumovirus (HMPV), human parainfluenza virus (HPIV), human respiratory syncytial virus (HRSV), and human rhinovirus (HRV). To ensure the accuracy of the analysis, we excluded viruses not detected by specific types of PCR tests, including HBoV, HCoV, HEV, and HRV. We only included viruses that were consistently tested across all types of RV-PCR tests, including HAdV, IFV, HMPV, HPIV, and HRSV. A patient infected with two or more viruses, including HAdV, was considered to be infected with HAdV, whereas a patient infected with two or more viruses other than HAdV was considered to be infected with each respective virus.
Korean Classification of Diseases (KCD)-8 codes related to ARD were selected based on the opinions of infectious medicine specialists (
Supplementary Table 2), and only health care use with selected KCD-8 codes was included in our analysis of the clinical outcomes of ARD. Furthermore, instances of healthcare utilization that occurred more than 7 days after the RV-PCR test were deemed unrelated to the test result and subsequently excluded from the analysis. Previous studies investigated adenovirus pneumonia in the Korean military have shown that the time from symptom onset to hospital admission is less than a week or about a week.
389 Moreover, it is generally challenging to obtain medical treatment at a military hospital while in service. Consequently, in most cases, individuals will visit a military hospital and undergo a PCR test if symptoms persist for about a week within their assigned unit. Based on these considerations, we defined that healthcare visits occurring after one week from the PCR test are less likely to be directly linked to the PCR test itself. Additionally, if the interval between two consecutive health care uses was 14 days or longer, the latter and subsequent health care uses were determined to be unrelated to the RV-PCR test result and were excluded from our analysis. In cases where the same patient received numerous RV-PCR tests, we reviewed the DEMIS thoroughly to determine whether those tests were for the same infectious case or not to avoid duplicate entries. The establishment of these protocols was guided by experts, including three pulmonologists who are currently working at the university hospitals.
The clinical outcomes of ARD, including healthcare utilization metrics (emergency visits, outpatient visits, hospitalization, ICU admission, and transfers) and prognosis indicators (treatment received, incidence of pneumonia, cases of severe ARD, and mortality), were thoroughly analyzed. We also assessed the number of patients who utilized healthcare services, along with the frequency or duration of visits for emergency, outpatient, hospitalization, and ICU. Additionally, we investigated transfers from military to civilian hospitals for advanced medical intervention. The treatment modalities analyzed included oxygen therapy, high flow nasal cannula (HFNC), MV, extracorporeal membrane oxygenation (ECMO), cidofovir, and continuous renal replacement therapy (CRRT). Pneumonia was defined using KCD-8 codes J12-J18. Severe ARD cases were defined as those receiving one or more of the following treatments: 1) HFNC, 2) MV, or 3) ECMO. We also collected data on patient mortality, if observed, to analyze the overall mortality rates associated with ARD.
Statistical analysis
We performed a descriptive analysis to examine the characteristics of the study participants. Chi-square tests, Fisher’s exact tests, and t-tests were conducted to compare the clinical outcomes between HAdV-infected and non-HAdV-infected patients. P values < 0.05 in a two-sided test were considered statistically significant. All statistical analyses were performed using R (version 4.3.0).
Ethics statement
This study was approved by the Institutional Review Board of Chung-Ang University (1041078-202208-HR-193) and the Armed Forces Medical Command (AFMC-202209-HR-046-01). The requirement for informed consent was waived.
RESULTS
From the DEMIS database, we retrieved a total of 23,830 patients who underwent PCR testing from January 2013 to July 2022, among whom, 10,670 (44.78%) tested positive.
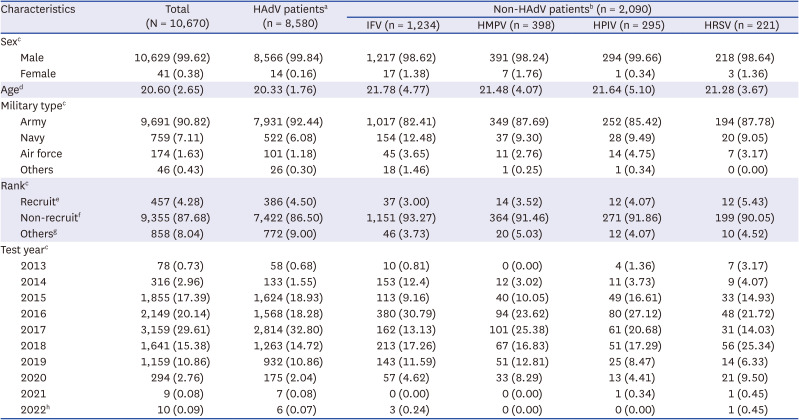
Table 1 illustrates the characteristics of the patients who tested positive for respiratory viruses in the Korean military during the study period. Among those who tested positive, 8,580 (80.41%) tested positive for HAdV. The study population consisted predominantly of male individuals, with HAdV being the most prevalent among males. The mean age of patients with HAdV was 20.33 (standard deviation 1.76) years. The first HAdV outbreak started in January 2015, and the peak positive rate was shown in March 2017. Since then, the HAdV outbreaks have continued sporadically (
Fig. 1).
Table 1
Characteristics of patients who tested positive for respiratory viruses in the Korean military, January 2013 to July 2022
|
Characteristics |
Total (N = 10,670) |
HAdV patientsa (n = 8,580) |
Non-HAdV patientsb (n = 2,090) |
|
IFV (n = 1,234) |
HMPV (n = 398) |
HPIV (n = 295) |
HRSV (n = 221) |
|
Sexc
|
|
|
|
|
|
|
|
Male |
10,629 (99.62) |
8,566 (99.84) |
1,217 (98.62) |
391 (98.24) |
294 (99.66) |
218 (98.64) |
|
Female |
41 (0.38) |
14 (0.16) |
17 (1.38) |
7 (1.76) |
1 (0.34) |
3 (1.36) |
|
Aged
|
20.60 (2.65) |
20.33 (1.76) |
21.78 (4.77) |
21.48 (4.07) |
21.64 (5.10) |
21.28 (3.67) |
|
Military typec
|
|
|
|
|
|
|
|
Army |
9,691 (90.82) |
7,931 (92.44) |
1,017 (82.41) |
349 (87.69) |
252 (85.42) |
194 (87.78) |
|
Navy |
759 (7.11) |
522 (6.08) |
154 (12.48) |
37 (9.30) |
28 (9.49) |
20 (9.05) |
|
Air force |
174 (1.63) |
101 (1.18) |
45 (3.65) |
11 (2.76) |
14 (4.75) |
7 (3.17) |
|
Others |
46 (0.43) |
26 (0.30) |
18 (1.46) |
1 (0.25) |
1 (0.34) |
0 (0.00) |
|
Rankc
|
|
|
|
|
|
|
|
Recruite
|
457 (4.28) |
386 (4.50) |
37 (3.00) |
14 (3.52) |
12 (4.07) |
12 (5.43) |
|
Non-recruitf
|
9,355 (87.68) |
7,422 (86.50) |
1,151 (93.27) |
364 (91.46) |
271 (91.86) |
199 (90.05) |
|
Othersg
|
858 (8.04) |
772 (9.00) |
46 (3.73) |
20 (5.03) |
12 (4.07) |
10 (4.52) |
|
Test yearc
|
|
|
|
|
|
|
|
2013 |
78 (0.73) |
58 (0.68) |
10 (0.81) |
0 (0.00) |
4 (1.36) |
7 (3.17) |
|
2014 |
316 (2.96) |
133 (1.55) |
153 (12.4) |
12 (3.02) |
11 (3.73) |
9 (4.07) |
|
2015 |
1,855 (17.39) |
1,624 (18.93) |
113 (9.16) |
40 (10.05) |
49 (16.61) |
33 (14.93) |
|
2016 |
2,149 (20.14) |
1,568 (18.28) |
380 (30.79) |
94 (23.62) |
80 (27.12) |
48 (21.72) |
|
2017 |
3,159 (29.61) |
2,814 (32.80) |
162 (13.13) |
101 (25.38) |
61 (20.68) |
31 (14.03) |
|
2018 |
1,641 (15.38) |
1,263 (14.72) |
213 (17.26) |
67 (16.83) |
51 (17.29) |
56 (25.34) |
|
2019 |
1,159 (10.86) |
932 (10.86) |
143 (11.59) |
51 (12.81) |
25 (8.47) |
14 (6.33) |
|
2020 |
294 (2.76) |
175 (2.04) |
57 (4.62) |
33 (8.29) |
13 (4.41) |
21 (9.50) |
|
2021 |
9 (0.08) |
7 (0.08) |
0 (0.00) |
0 (0.00) |
1 (0.34) |
1 (0.45) |
|
2022h
|
10 (0.09) |
6 (0.07) |
3 (0.24) |
0 (0.00) |
0 (0.00) |
1 (0.45) |
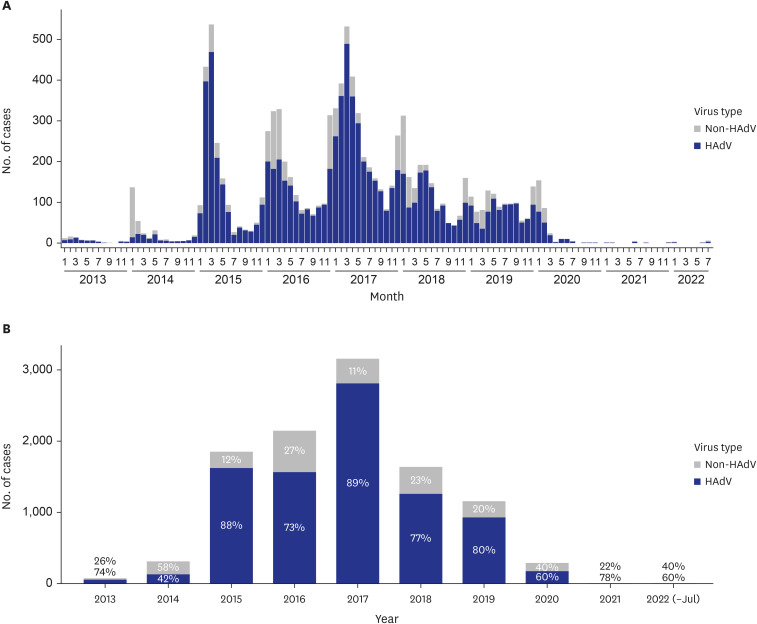
Fig. 1
Proportion of HAdV infections among acute respiratory virus infections in the Korean military from January 2013 to July 2022. The height of each bar corresponds to the number of infections observed in each month. The blue bars indicate the number of HAdV infections, and the grey bars represent the number of non-HAdV infections, which include influenza virus, human metapneumovirus, human parainfluenza virus, and human respiratory syncytial virus. (A) Monthly trend in the number of HAdV and non-HAdV infections. (B) Yearly trend in the number of HAdV and non-HAdV infections with percentages.
HAdV = human adenovirus.
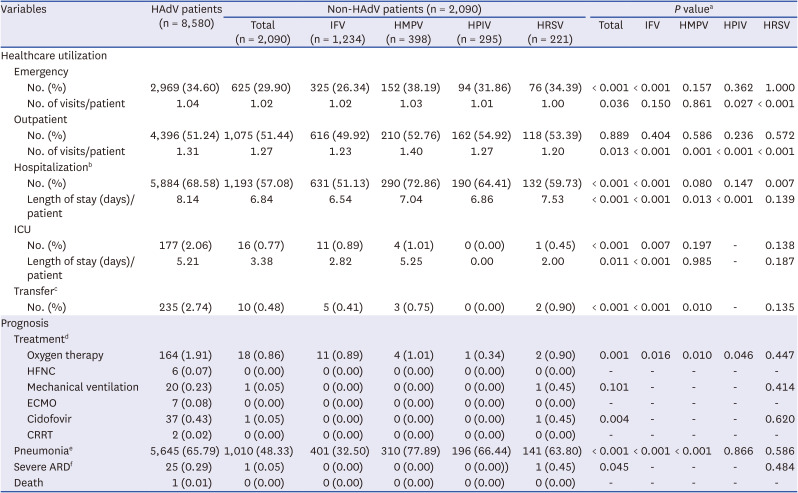
The clinical outcomes, including healthcare utilization and prognosis, between HAdV- and non-HAdV-infected patients are summarized in
Table 2. HAdV-infected patients were more likely to visit emergency (34.60% vs. 29.90%) and to be hospitalized (68.58% vs. 57.08%) compared to non-HAdV-infected patients. The number of visits to emergency (1.04 vs. 1.02) or outpatient clinic (1.31 vs. 1.27) and the duration of hospitalization (8.14 days vs. 6.84 days) per patient were higher among the HAdV-infected patients compared to non-HAdV-infected patients. The rate of transferred cases to civilian hospitals was higher among the HAdV-infected patients (2.74% vs. 0.48%). We also present a comparison of the prognosis between patients infected with HAdV and with non-HAdV. The percentage of patients receiving oxygen therapy was higher in HAdV-infected patients compared to non-HAdV-infected patients (1.91% vs. 0.86%). Among HAdV patients, 164 (1.91%) received oxygen therapy, 6 (0.07%) received HFNC, 20 (0.23%) underwent MV, 7 (0.08%) received ECMO, 37 (0.43%) received cidofovir, and 2 (0.02%) underwent CRRT. In contrast, among non-HAdV patients, 18 (0.86%) received oxygen therapy, only one (0.05%) received MV, and none received HFNC, ECMO, or CRRT. Additionally, a higher proportion of HAdV-infected patients (65.79%) presented with pneumonia compared to those infected with non-HAdV (48.33%). When focusing exclusively on viral pneumonia (J12) and unspecified pneumonia (J18), a significant proportion (62.82%) of HAdV-infected patients still sought healthcare at least once due to instances of viral or unspecified pneumonia. Moreover, a higher proportion of HAdV infected patients experienced progression to severe ARD (0.29% vs. 0.05%). Lastly, one case of death was observed among patients infected with HAdV, whereas no cases of death were observed in non-HAdV infected patients. When we examined non-HAdV infected patients by virus type, it became evident that clinical outcomes were generally less favorable in patients infected with HAdV compared to those infected with non-HAdV, except in cases involving HMPV infection. Additionally, we divided patients infected with only HAdV and patients co-infected with HAdV and other viruses. Upon comparing the clinical outcomes of the two groups, we confirmed that there was no significant difference between patients infected with only HAdV and patients co-infected with HAdV and other viruses (
Supplementary Tables 3 and
4).
Table 2
Comparison of clinical outcomes between HAdV and non-HAdV patients
|
Variables |
HAdV patients (n = 8,580) |
Non-HAdV patients (n = 2,090) |
P valuea
|
|
Total (n = 2,090) |
IFV (n = 1,234) |
HMPV (n = 398) |
HPIV (n = 295) |
HRSV (n = 221) |
Total |
IFV |
HMPV |
HPIV |
HRSV |
|
Healthcare utilization |
|
|
|
|
|
|
|
|
|
|
|
|
Emergency |
|
|
|
|
|
|
|
|
|
|
|
|
|
No. (%) |
2,969 (34.60) |
625 (29.90) |
325 (26.34) |
152 (38.19) |
94 (31.86) |
76 (34.39) |
< 0.001 |
< 0.001 |
0.157 |
0.362 |
1.000 |
|
|
No. of visits/patient |
1.04 |
1.02 |
1.02 |
1.03 |
1.01 |
1.00 |
0.036 |
0.150 |
0.861 |
0.027 |
< 0.001 |
|
Outpatient |
|
|
|
|
|
|
|
|
|
|
|
|
|
No. (%) |
4,396 (51.24) |
1,075 (51.44) |
616 (49.92) |
210 (52.76) |
162 (54.92) |
118 (53.39) |
0.889 |
0.404 |
0.586 |
0.236 |
0.572 |
|
|
No. of visits/patient |
1.31 |
1.27 |
1.23 |
1.40 |
1.27 |
1.20 |
0.013 |
< 0.001 |
0.001 |
< 0.001 |
< 0.001 |
|
Hospitalizationb
|
|
|
|
|
|
|
|
|
|
|
|
|
|
No. (%) |
5,884 (68.58) |
1,193 (57.08) |
631 (51.13) |
290 (72.86) |
190 (64.41) |
132 (59.73) |
< 0.001 |
< 0.001 |
0.080 |
0.147 |
0.007 |
|
|
Length of stay (days)/patient |
8.14 |
6.84 |
6.54 |
7.04 |
6.86 |
7.53 |
< 0.001 |
< 0.001 |
0.013 |
< 0.001 |
0.139 |
|
ICU |
|
|
|
|
|
|
|
|
|
|
|
|
|
No. (%) |
177 (2.06) |
16 (0.77) |
11 (0.89) |
4 (1.01) |
0 (0.00) |
1 (0.45) |
< 0.001 |
0.007 |
0.197 |
- |
0.138 |
|
|
Length of stay (days)/patient |
5.21 |
3.38 |
2.82 |
5.25 |
0.00 |
2.00 |
0.011 |
< 0.001 |
0.985 |
- |
0.187 |
|
Transferc
|
|
|
|
|
|
|
|
|
|
|
|
|
|
No. (%) |
235 (2.74) |
10 (0.48) |
5 (0.41) |
3 (0.75) |
0 (0.00) |
2 (0.90) |
< 0.001 |
< 0.001 |
0.010 |
- |
0.135 |
|
Prognosis |
|
|
|
|
|
|
|
|
|
|
|
|
Treatmentd
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Oxygen therapy |
164 (1.91) |
18 (0.86) |
11 (0.89) |
4 (1.01) |
1 (0.34) |
2 (0.90) |
0.001 |
0.016 |
0.010 |
0.046 |
0.447 |
|
|
HFNC |
6 (0.07) |
0 (0.00) |
0 (0.00) |
0 (0.00) |
0 (0.00) |
0 (0.00) |
- |
- |
- |
- |
- |
|
|
Mechanical ventilation |
20 (0.23) |
1 (0.05) |
0 (0.00) |
0 (0.00) |
0 (0.00) |
1 (0.45) |
0.101 |
- |
- |
- |
0.414 |
|
|
ECMO |
7 (0.08) |
0 (0.00) |
0 (0.00) |
0 (0.00) |
0 (0.00) |
0 (0.00) |
- |
- |
- |
- |
- |
|
|
Cidofovir |
37 (0.43) |
1 (0.05) |
0 (0.00) |
0 (0.00) |
0 (0.00) |
1 (0.45) |
0.004 |
- |
- |
- |
0.620 |
|
|
CRRT |
2 (0.02) |
0 (0.00) |
0 (0.00) |
0 (0.00) |
0 (0.00) |
0 (0.00) |
- |
- |
- |
- |
- |
|
Pneumoniae
|
5,645 (65.79) |
1,010 (48.33) |
401 (32.50) |
310 (77.89) |
196 (66.44) |
141 (63.80) |
< 0.001 |
< 0.001 |
< 0.001 |
0.866 |
0.586 |
|
Severe ARDf
|
25 (0.29) |
1 (0.05) |
0 (0.00) |
0 (0.00) |
0 (0.00)) |
1 (0.45) |
0.045 |
- |
- |
- |
0.484 |
|
Death |
1 (0.01) |
0 (0.00) |
0 (0.00) |
0 (0.00) |
0 (0.00) |
0 (0.00) |
- |
- |
- |
- |
- |
DISCUSSION
Our findings revealed that HAdV-infected Korean military patients demonstrated significantly higher disease severity compared to those infected with non-HAdV viruses. Our analysis of 10,670 respiratory virus-positive patients in the Korean military from January 2013 to July 2022 revealed that HAdV infection was associated with increased healthcare utilization, including emergency visits, outpatient clinics visits, hospitalizations, and ICU admissions, as well as increased likelihood of transfer to civilian hospitals. Additionally, a higher proportion of HAdV-infected patients required interventions such as oxygen therapy, and presented with pneumonia. These findings underscore the significance of HAdV infections in terms of their impact on disease severity in a military setting and emphasize the need for prevention and appropriate management strategies for HAdV-associated ARD.
To compare the burden of HAdV-associated ARD between military and civilian populations, we analyzed data from the acute respiratory infection surveillance system from January 2015 to August 2023 (
Supplementary Fig. 1). The acute respiratory infection surveillance system, established by the Korea Centers for Disease Control and Prevention, provides statistical information weekly using data reported on its website. The nature of the surveillance data does not enable us to determine the overall number of infections within the civilian population. However, when comparing the infection rates of HAdV to those of other viruses, it became evident that there was a significantly higher proportion of HAdV infections within the military population compared to the civilian population. The surveillance system does not report data with stratification by age groups, which results in the inclusion of cases across all age groups. Consequently, it should be acknowledged that the actual infection rates in the civilian population within the specific age group comparable to our study population might be lower, which may lead to even greater disparities within the same age groups.
As shown in
Fig. 1 and
Supplementary Fig. 1, respiratory infections, including HAdV cases, showed an overall decrease during the COVID-19 pandemic era because of infection control measures such as mask-wearing, social distancing, and hand hygiene. However, since April 2023, there has been a significant increase in the number of HAdV infection cases reported. Indeed, several recent studies have also observed a resurgence of various respiratory infections, including RSV and influenza, after relaxing non-pharmaceutical interventions against COVID-19 in various settings.
101112 Given the continuous intake of new recruits into the military, a resurgence of ARD caused by adenovirus could be plausible in the post-COVID-19 era, even though it remains uncertain whether the resurgence of HAdV-55, known for causing more severe health outcomes, will occur. The possible resurgence of HAdV in the military can also be supported by the fact that the temporary discontinuation of the vaccine in 1996 led to a rapid resurgence of HAdV infection rates in the USA.
13 Moreover, a comprehensive systematic review conducted in China revealed that among adolescents and adults (including military population) infected with HAdV, 21.7% and 25.0% of each group, respectively, acquired pneumonia due to HAdV infection.
14 In contrast, our study found that a higher proportion of HAdV-infected patients (65.79%) developed pneumonia, suggesting that the military population faces an increased risk not only of HAdV-associated ARD but also of HAdV-associated pneumonia. Therefore, it is necessary to implement effective infection control measures within military training centers.
In 2010, HAdV-55, a new variant resulting from the recombination of types 11 and 14, formerly referred to as type 11a, was named type 55. Since 2005, a series of outbreaks caused by HAdV-55 have been reported in various countries, including China,
1516 Singapore,
17 and Turkey.
18 In China, such outbreaks have not been limited to military groups but have also been observed among the general population, including schools and sports facilities.
192021 In Korea, the first case of HAdV-55 pneumonia was discovered in 2012, and the results of previous studies have suggested that HAdV-55 is the predominant causative agent of ARD in the Korean military.
45722 Also, several studies have shown that HAdV-55 results in a poorer prognosis than other HAdV serotypes.
4 However, we could not perform molecular typing of HAdV in this study, and further research incorporating molecular typing analysis is needed to gain a more comprehensive understanding.
Factors such as close living conditions, intensive training, and a significant level of stress increase the risk of HAdV-associated ARD in the military population. Upon comparing prevalence rates across military types, a higher number of cases of HAdV infection were observed within the Army as opposed to the Navy and Air Force, as presented in
Table 1. Army personnel are exposed to conditions that facilitate close-contact scenarios, a known risk factor for infections, due to their larger population and extensive training facilities. Additionally, the expedited modernization initiative transitioning from shared open bay barracks housing, accommodating 30–40 soldiers per room, to dormitory-style housing, with 8–10 soldiers per room, was accomplished more promptly in the Navy and Air Force. This achievement likely contributed to a more effective reduction in the close-contact environment, possibly explaining the variance in HAdV infection rates.
The high prevalence of infectious diseases in the military commonly leads to the reduction or cancellation of ongoing military training to mitigate outbreaks, ultimately resulting in a deterioration of military security capabilities. HAdV has a basic reproduction number of 2.34, which is similar to that of COVID-19 (2.84).
2324 In other words, HAdV is at risk of spreading as quickly as COVID-19 within densely populated settings such as the military. Our study showed that the average number of emergency visits was 1.04, whereas that of outpatient visits was 1.31. The average length of hospitalization and ICU stay was 8.14 and 5.21 days, respectively. Therefore, if a patient with HAdV is hospitalized after visiting the outpatient clinic or emergency room, the patient cannot complete the scheduled training for more than 8 days on average. The increasing incidence of HAdV infections leading to missed training sessions among patients may negatively impact national combat power and subsequently affect national security, while also necessitating additional defense expenditure for patients to reattempt incomplete training. Furthermore, an outbreak of HAdV in the military may cause severe morbidity and mortality among young people in their 20s who are conscripted in accordance with mandatory military service. Therefore, the government is responsible for protecting the right to the health of soldiers so that they can safely fulfill their duty.
Currently, there is no established treatment for HAdV, and vaccination is the most effective prevention method for viral diseases for which there is no treatment or treatment is rare. However, despite the ongoing outbreak in the military population, the development of HAdV vaccines by pharmaceutical companies is limited, which is primarily attributed to the perceived lack of commercial viability, highlighting the need for government-led initiatives to advance vaccine development efforts. To date, two studies conducted in China have attempted to identify neutralizing antibodies against various types of HAdV.
2526 In Korea, a total of four preclinical studies have been conducted, encompassing analysis of the cross-protection effects among HAdV genotypes, the standardization of plaque assays for neutralizing antibodies against HAdV types, the acquisition of vaccine development candidate materials, and the process of patent deposition.
27 Considering the burden of HAdV in a military setting, it is necessary to continue ongoing research endeavors to develop a vaccine for HAdV.
Our study has some limitations that warrant discussion. First, we were unable to conduct molecular typing to identify the specific types of HAdV responsible for the issue in question. However, evidence from previous studies and reported data suggest that ongoing HAdV outbreaks are predominantly caused by HAdV-55. Second, during the study period, Korean military hospitals used four types of RV-PCR tests, each with varying numbers of included viruses. As a result, the types of respiratory viruses considered in our analysis were limited. Third, as we did not have bacterial PCR test results and administration of antivirals or antibiotics other than cidofovir in our dataset, they were not included in our analysis. Moreover, not all infected individuals underwent PCR testing, which introduces the possibility of underestimating the actual extent of infection based on the constructed data. Indeed, as shown in
Supplementary Table 5, upon reviewing data from the Army, Navy, and Air Force headquarters on individuals who received treatment for HAdV-associated pneumonia at civilian hospitals, we identified 51 additional patients who were excluded from our study population because they did not undergo PCR testing at military hospitals. By reviewing their clinical outcomes and prognoses, we confirmed more severe ARD cases associated with HAdV infection, including two more deaths. Moreover, according to a previous study, there were five deaths due to HAdV infection in the Korean military from 2013 to 2018.
6 Consequently, the true burden of disease due to HAdV-associated ARD in the Korean military may be greater than we have estimated. Finally, when tracking health care use records, we applied the following specific criteria: the first visit had to occur within 7 days after the PCR test date, and consecutive health care use visits had to occur within a 14-day interval. This selection process may have resulted in the exclusion of certain healthcare use records, leading to potential underestimation of actual healthcare use.
Nonetheless, this study represents an updated analysis of a previous nationwide epidemiologic study on the ARD outbreak status within the Korean military. It stands as the most comprehensive analysis of the ARD status within the Korean military population to date, encompassing the longest study period and including all patients who underwent PCR testing in Korean military hospitals. Furthermore, a comparative assessment of the prognosis between HAdV-associated ARD and non-HAdV-associated ARD was conducted, the results of which enhance our understanding of the clinical outcomes associated with different types of ARDs within the military population. Finally, we were able to track patients who were transferred to the civilian hospital for advanced treatments by collecting data from the Ministry of National Defense, Army Headquarters, Navy Headquarters, Air Force Headquarters, and Armed Forces Medical Command.
Prior to the COVID-19 pandemic, the Korean military has experienced recurrent outbreaks of HAdV, leading to a heightened prevalence of severe ARD and unfavorable clinical outcomes. To effectively control this ongoing outbreak, it is crucial to intensify efforts in outbreak management, including education, environmental improvements, and the development of HAdV vaccines that are specific to the most predominant serotype in the Korean military. Additionally, to minimize the impact of future infectious disease outbreaks, it is essential to ensure the availability of adequate vaccine development technology for domestic production in Korea. This proactive measure would serve to enhance preparedness and response capabilities, ultimately reducing the potential damage caused by infectious disease outbreaks in the future.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download