INTRODUCTION
Mpox is a viral illness caused by the monkeypox virus, which belongs to the family Poxviridae, subfamily Chordopoxvirinae, and genus orthopoxvirus, which results in a characteristic skin rash.
1 The first human case was reported in the Democratic Republic of the Congo in 1970.
2 Human-to-human transmission can occur via respiratory secretions, direct contact including sexual contact, vertical transmission, percutaneous transmission, and indirect contact through fomites.
3 Mpox was primarily an endemic disease in Africa, but global outbreaks occurred in non-endemic countries in May 2022.
4 The first case was confirmed in South Korea in June 2022,
5 and more than 100 patients have been identified.
6 Although there are a few case reports,
5789 data summarizing the clinical features of Mpox patients in South Korea are lacking. In this study, we systematically summarize the clinical features of South Korean Mpox patients to improve understanding of Mpox and to facilitate clinical practice.
METHODS
Study design and participants
This multicenter retrospective study was performed with patients diagnosed with Mpox in South Korea between June 1, 2022 and May 26, 2023. All adult patients who were admitted to the National Medical Center, Seoul Medical Center, Seoul National University Hospital, or Seoul National University Bundang Hospital and were diagnosed with Mpox and discharged by June 30, 2023 were reviewed.
Microbiological data
Serological test for Treponema pallidum infection were done with a Rapid Plasma Regain (RPR) test reported with a quantitative titer using Asan RPR Card Test (Asan Pharm. Co., Ltd, Seoul, Korea) or Macro-Vue RPR card test (Becton Dickinson BD Microbiology Systems, Sparks, MD, USA).
HIV antigen/antibody test was performed using the ARCHITECT i2000SR and ARCHITECT HIV Ag/Ab Combo assay (Abbott, Chicago, IL, USA). For patients previously diagnosed with human immunodeficiency virus type 1 (HIV-1) infection, HIV-1 RNA test was conducted using the Alinity m and Alinity m HIV-1 AMP Kit (Abbott).
Definitions
A case of Mpox was defined as a laboratory-confirmed monkeypox virus infection by a positive result in a polymerase chain reaction (PCR) assay of any specimens (skin, oropharyngeal swab, blood, and others). PCR assay was performed as previously described.
5 Viral DNAs were extracted from specimens of patients by using the QIAamp DNA blood Mini Kit (QIAGEN, Crawley, UK) and real-time PCR assays specific to the monkeypox virus were performed with the viral DNAs using a laboratory-developed real-time PCR kit.
Demographic characteristics, underlying immunocompromising diseases and conditions, sexual orientation, history of Jynneos (Modified Vaccinia Ankara, Bavarian Nordic) vaccination, possible route of transmission, baseline laboratory data, clinical symptoms, patterns of skin rash, co-infection, treatment, and outcome were reviewed. Sexual orientation was categorized as 1) homosexual or bisexual, and 2) unknown. To detect concomitant sexually transmitted infections (STIs), PCR was performed using urine samples for males and vaginal samples for females. The antiviral medication used to treat Mpox was investigated, and concurrent medications were also reviewed, including 1) drugs previously used for underlying immunocompromising diseases, 2) treatment for sexually transmitted diseases, and 3) antibiotics for skin lesions. To investigate the possible route of transmission, recent sexual contact and travel history within the past 3 weeks were reviewed. Severe Mpox was defined as previously described.
10
Statistical analysis
The χ2 or Fisher’s exact test was used to compare categorical variables, and Student’s t-test or the Mann-Whitney U test was used to compare continuous variables, as appropriate. All tests of significance were two-tailed, and P values < 0.05 were considered to indicate statistical significance. Data analyses was performed using R version 4.1.3 (R Project for Statistical Computing, Vienna, Austria).
Ethics statement
This study was approved by the Institutional Review Board of each participating institution (NMC-2023-06-066, SNUH-2307-042-1448, SNUBH-2308-845-405, SMC-2023-07-001). All participants were given informed consent.
RESULTS
Demographic and baseline characteristics of the patients
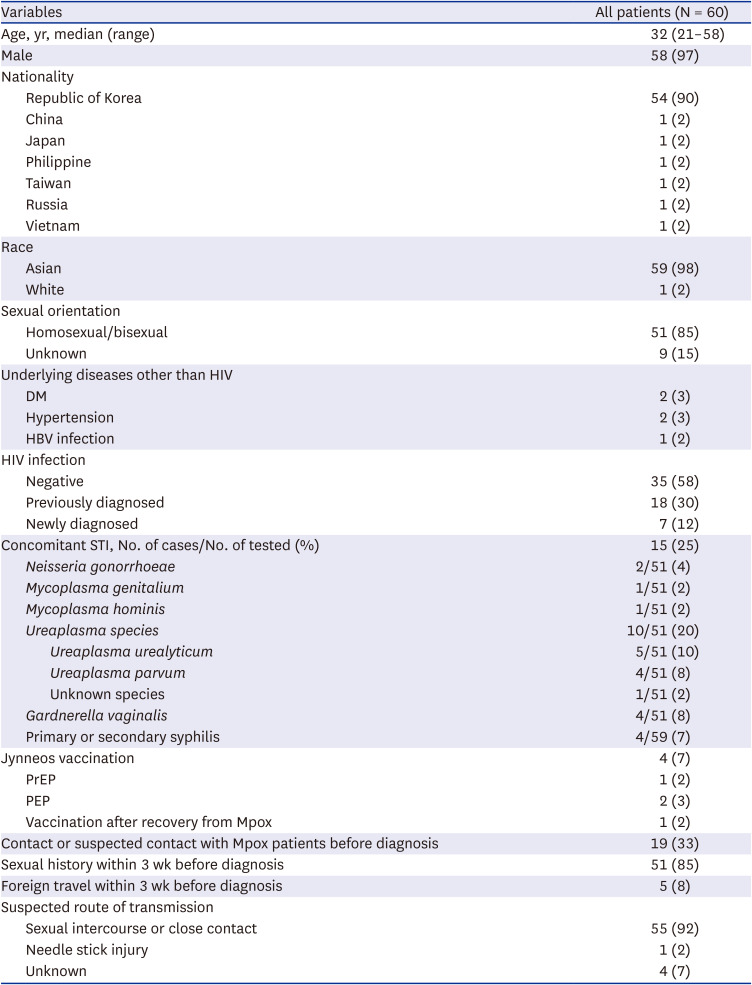
Sixty patients were identified between June 1, 2022 and May 26, 2023, accounting for 65.9% (60/91) of the Mpox cases reported in South Korea during this period. The demographic and baseline characteristics of the patients are presented in
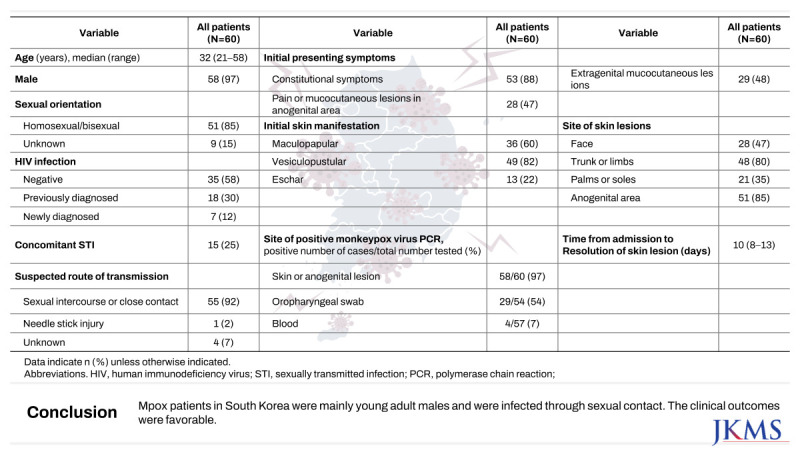
Table 1. Median age was 32 years (range, 21−58 years) and 58 (97%) patients were male. Overall, 51 (85%) patients reported their sexual orientation as homosexual or bisexual. The sexual orientation of nine (15%) patients was not clearly documented.
Table 1
Demographic and baseline characteristics of the patients
|
Variables |
All patients (N = 60) |
|
Age, yr, median (range) |
32 (21–58) |
|
Male |
58 (97) |
|
Nationality |
|
|
Republic of Korea |
54 (90) |
|
China |
1 (2) |
|
Japan |
1 (2) |
|
Philippine |
1 (2) |
|
Taiwan |
1 (2) |
|
Russia |
1 (2) |
|
Vietnam |
1 (2) |
|
Race |
|
|
Asian |
59 (98) |
|
White |
1 (2) |
|
Sexual orientation |
|
|
Homosexual/bisexual |
51 (85) |
|
Unknown |
9 (15) |
|
Underlying diseases other than HIV |
|
|
DM |
2 (3) |
|
Hypertension |
2 (3) |
|
HBV infection |
1 (2) |
|
HIV infection |
|
|
Negative |
35 (58) |
|
Previously diagnosed |
18 (30) |
|
Newly diagnosed |
7 (12) |
|
Concomitant STI, No. of cases/No. of tested (%) |
15 (25) |
|
Neisseria gonorrhoeae
|
2/51 (4) |
|
Mycoplasma genitalium
|
1/51 (2) |
|
Mycoplasma hominis
|
1/51 (2) |
|
Ureaplasma species
|
10/51 (20) |
|
|
Ureaplasma urealyticum
|
5/51 (10) |
|
|
Ureaplasma parvum
|
4/51 (8) |
|
|
Unknown species |
1/51 (2) |
|
Gardnerella vaginalis
|
4/51 (8) |
|
Primary or secondary syphilis |
4/59 (7) |
|
Jynneos vaccination |
4 (7) |
|
PrEP |
1 (2) |
|
PEP |
2 (3) |
|
Vaccination after recovery from Mpox |
1 (2) |
|
Contact or suspected contact with Mpox patients before diagnosis |
19 (33) |
|
Sexual history within 3 wk before diagnosis |
51 (85) |
|
Foreign travel within 3 wk before diagnosis |
5 (8) |
|
Suspected route of transmission |
|
|
Sexual intercourse or close contact |
55 (92) |
|
Needle stick injury |
1 (2) |
|
Unknown |
4 (7) |
Concomitant STIs were also investigated. In total, 24% (12/51) of patients had positive PCR results for STIs, including Neisseria gonorrhoeae, Mycoplasma genitalium, Mycoplasma hominis, Ureaplasma species, Gardnerella vaginalis, and T. pallidum. Among ten patients with positive serology for syphilis, four were treated with benzathine penicillin G during hospitalization, with two diagnosed with primary syphilis and two with secondary syphilis. The remaining six were considered to have a serofast status with a previous history of syphilis treatment.
Four (7%) patients received Mpox vaccination. Among them, one (2%) was vaccinated prior to monkeypox virus infection, two (3%) were vaccinated for postexposure prophylaxis, and one (2%) was vaccinated after recovery. The patient who received vaccination before infection was diagnosed 5 days after receiving a single dose.
Concerning epidemiological associations, 19 (33%) patients reported having contact with an individual diagnosed with Mpox or suspected of having Mpox prior to their diagnosis. Interval between suspected exposure to Mpox patient and initial symptom onset was 7 days (interquartile range [IQR], 3−11) (
Supplementary Fig. 1). Fifty-one (85%) patients had a history of sexual intercourse within 3 weeks of diagnosis and five (8%) patients had a history of foreign travel, including to Germany, the United Kingdom, the United Arab Emirates, Taiwan, and Japan. Overall, 55 (92%) patients were presumed to have been infected through sexual or close contact, one (2%) patient had a history of needle stick injury from Mpox patients, and a clear route of transmission was not identified for four (7%) patients. All patients included in this study received diagnostic test due to symptom onset.
Clinical manifestations, diagnosis, and treatment of the patients
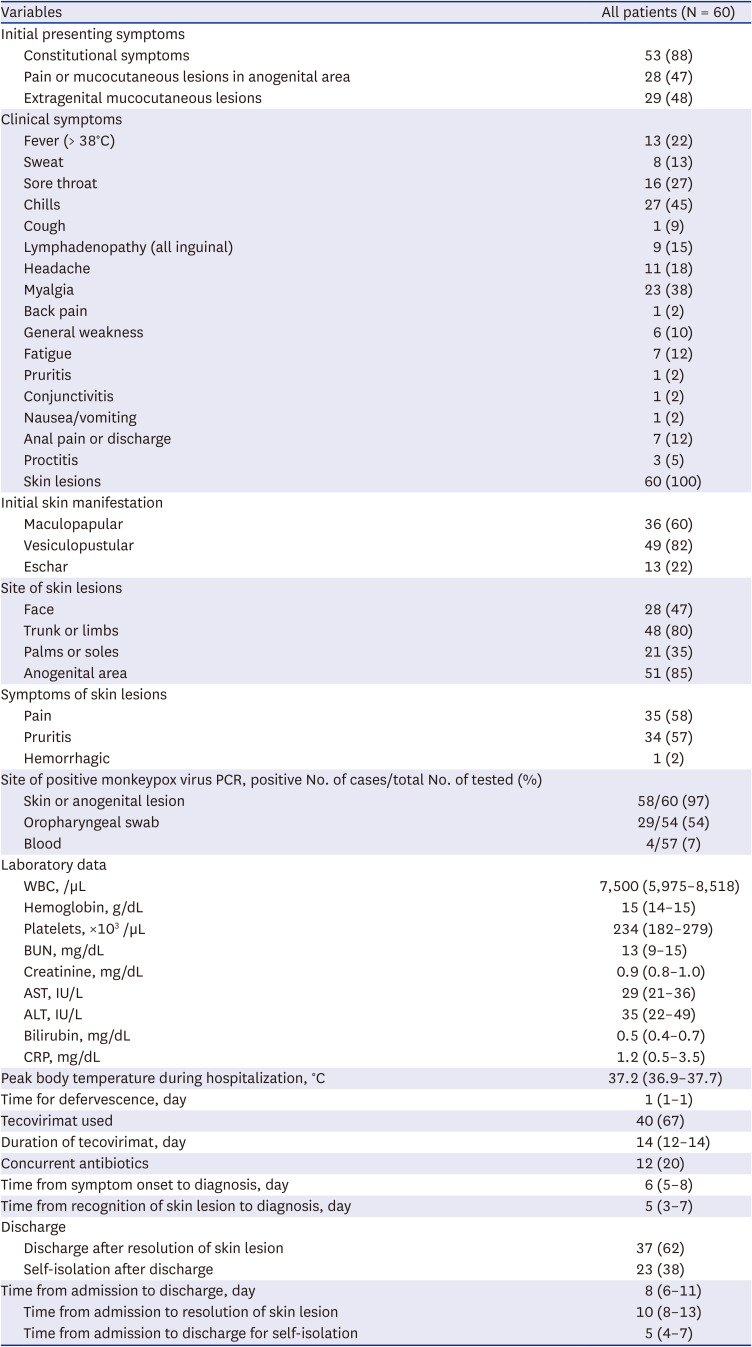
The clinical manifestations of the patients are presented in
Table 2. The initial presenting symptoms were predominantly constitutional symptoms (88%), and anogenital and extragenital mucocutaneous lesions were identified in 47% and 48% of patients, respectively. The most common clinical symptom other than skin lesions was chills (45%) followed by myalgia (38%), sore throat (27%), and fever of 38°C or higher (22%). Seven (12%) patients reported anal pain or discharge and three (5%) patients had proctitis. Skin lesions were observed in all patients at initial diagnosis and predominantly presented as vesiculopustular lesions (82%), followed by maculopapular lesions (60%) and eschar (22%). The most common anatomical site for skin lesions was the anogenital region (85%), followed by the trunk or limbs (80%).
Table 2
Clinical manifestation, diagnosis, and treatment of the patients
|
Variables |
All patients (N = 60) |
|
Initial presenting symptoms |
|
|
Constitutional symptoms |
53 (88) |
|
Pain or mucocutaneous lesions in anogenital area |
28 (47) |
|
Extragenital mucocutaneous lesions |
29 (48) |
|
Clinical symptoms |
|
|
Fever (> 38°C) |
13 (22) |
|
Sweat |
8 (13) |
|
Sore throat |
16 (27) |
|
Chills |
27 (45) |
|
Cough |
1 (9) |
|
Lymphadenopathy (all inguinal) |
9 (15) |
|
Headache |
11 (18) |
|
Myalgia |
23 (38) |
|
Back pain |
1 (2) |
|
General weakness |
6 (10) |
|
Fatigue |
7 (12) |
|
Pruritis |
1 (2) |
|
Conjunctivitis |
1 (2) |
|
Nausea/vomiting |
1 (2) |
|
Anal pain or discharge |
7 (12) |
|
Proctitis |
3 (5) |
|
Skin lesions |
60 (100) |
|
Initial skin manifestation |
|
|
Maculopapular |
36 (60) |
|
Vesiculopustular |
49 (82) |
|
Eschar |
13 (22) |
|
Site of skin lesions |
|
|
Face |
28 (47) |
|
Trunk or limbs |
48 (80) |
|
Palms or soles |
21 (35) |
|
Anogenital area |
51 (85) |
|
Symptoms of skin lesions |
|
|
Pain |
35 (58) |
|
Pruritis |
34 (57) |
|
Hemorrhagic |
1 (2) |
|
Site of positive monkeypox virus PCR, positive No. of cases/total No. of tested (%) |
|
|
Skin or anogenital lesion |
58/60 (97) |
|
Oropharyngeal swab |
29/54 (54) |
|
Blood |
4/57 (7) |
|
Laboratory data |
|
|
WBC, /µL |
7,500 (5,975–8,518) |
|
Hemoglobin, g/dL |
15 (14–15) |
|
Platelets, ×103 /µL |
234 (182–279) |
|
BUN, mg/dL |
13 (9–15) |
|
Creatinine, mg/dL |
0.9 (0.8–1.0) |
|
AST, IU/L |
29 (21–36) |
|
ALT, IU/L |
35 (22–49) |
|
Bilirubin, mg/dL |
0.5 (0.4–0.7) |
|
CRP, mg/dL |
1.2 (0.5–3.5) |
|
Peak body temperature during hospitalization, °C |
37.2 (36.9–37.7) |
|
Time for defervescence, day |
1 (1–1) |
|
Tecovirimat used |
40 (67) |
|
Duration of tecovirimat, day |
14 (12–14) |
|
Concurrent antibiotics |
12 (20) |
|
Time from symptom onset to diagnosis, day |
6 (5–8) |
|
Time from recognition of skin lesion to diagnosis, day |
5 (3–7) |
|
Discharge |
|
|
Discharge after resolution of skin lesion |
37 (62) |
|
Self-isolation after discharge |
23 (38) |
|
Time from admission to discharge, day |
8 (6–11) |
|
Time from admission to resolution of skin lesion |
10 (8–13) |
|
Time from admission to discharge for self-isolation |
5 (4–7) |
Specimens for diagnosis were most frequently collected from skin or anogenital lesions (100%), with a positivity rate of 97%. Oropharyngeal swabs had a positivity rate of 54% (29/54). By contrast, blood PCR tests had a lower positivity rate of 7% (4/57). The median time from any symptom onset to diagnosis was 6 days (IQR, 5−8 days) and the median time from skin manifestation to diagnosis was 5 days (IQR, 3−7 days).
During hospitalization, the peak body temperature recorded was a median of 37.2°C (IQR, 36.9−37.7°C), with fever lasting a median of 1 day (IQR, 1−1 day). The median white blood cell count was 7,500/µL, the median hemoglobin level was 15 g/dL, and the median platelet count was 234,000/µL without distinct cytopenia. The median C-reactive protein level was 1.2 mg/dL, indicating inflammatory markers were not significantly elevated.
For Mpox-specific treatment, tecovirimat was used exclusively. Forty (67%) patients received tecovirimat for a median duration of 14 days (IQR, 12−14 days). Additionally, 15 (25%) patients received concurrent antibiotics for co-existing STIs or wound infections due to skin lesions.
The median duration from hospital admission to the resolution of skin lesions was 10 days (IQR, 8−13 days). Overall, 37 (62%) patients were discharged after the resolution of skin lesions, while the other 23 (38%) patients were discharged before the resolution of skin lesions and self-isolated.
Severe manifestations of Mpox were identified in two patients, one with urethritis and the other with severe vaginal pain. There were no reported deaths.
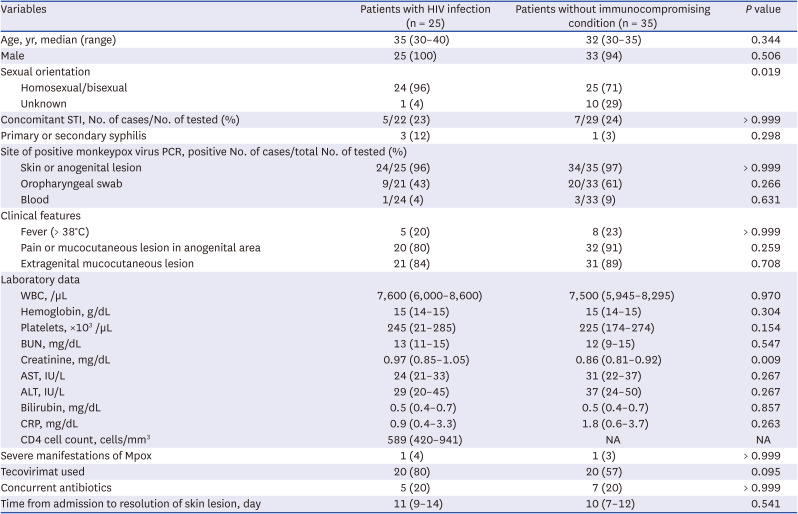
Demographic and clinical characteristics of the patients with HIV infection
Only HIV infection was identified as an underlying immunocompromising disease or condition. Of the 60 patients, 25 (42%) had HIV infection, of whom 18 (88%) had been previously diagnosed and 7 (12%) were newly diagnosed at admission. All patients who had been diagnosed with HIV infection before monkeypox virus infection were on antiretroviral therapy (ART) and maintained well-controlled HIV infection. All the patients on ART had an HIV viral load of < 20 copies/mL. We additionally investigated the baseline characteristics and clinical courses of the HIV patients. There was no significant difference between HIV patients and those without underlying immunocompromising conditions (
Table 3).
Table 3
Baseline and clinical characteristics of the patients with HIV infection and those without immunocompromising conditions
|
Variables |
Patients with HIV infection (n = 25) |
Patients without immunocompromising condition (n = 35) |
P value |
|
Age, yr, median (range) |
35 (30–40) |
32 (30–35) |
0.344 |
|
Male |
25 (100) |
33 (94) |
0.506 |
|
Sexual orientation |
|
|
0.019 |
|
Homosexual/bisexual |
24 (96) |
25 (71) |
|
Unknown |
1 (4) |
10 (29) |
|
Concomitant STI, No. of cases/No. of tested (%) |
5/22 (23) |
7/29 (24) |
> 0.999 |
|
Primary or secondary syphilis |
3 (12) |
1 (3) |
0.298 |
|
Site of positive monkeypox virus PCR, positive No. of cases/total No. of tested (%) |
|
|
|
|
Skin or anogenital lesion |
24/25 (96) |
34/35 (97) |
> 0.999 |
|
Oropharyngeal swab |
9/21 (43) |
20/33 (61) |
0.266 |
|
Blood |
1/24 (4) |
3/33 (9) |
0.631 |
|
Clinical features |
|
|
|
|
Fever (> 38°C) |
5 (20) |
8 (23) |
> 0.999 |
|
Pain or mucocutaneous lesion in anogenital area |
20 (80) |
32 (91) |
0.259 |
|
Extragenital mucocutaneous lesion |
21 (84) |
31 (89) |
0.708 |
|
Laboratory data |
|
|
|
|
WBC, /µL |
7,600 (6,000–8,600) |
7,500 (5,945–8,295) |
0.970 |
|
Hemoglobin, g/dL |
15 (14–15) |
15 (14–15) |
0.304 |
|
Platelets, ×103 /µL |
245 (21–285) |
225 (174–274) |
0.154 |
|
BUN, mg/dL |
13 (11–15) |
12 (9–15) |
0.547 |
|
Creatinine, mg/dL |
0.97 (0.85–1.05) |
0.86 (0.81–0.92) |
0.009 |
|
AST, IU/L |
24 (21–33) |
31 (22–37) |
0.267 |
|
ALT, IU/L |
29 (20–45) |
37 (24–50) |
0.267 |
|
Bilirubin, mg/dL |
0.5 (0.4–0.7) |
0.5 (0.4–0.7) |
0.857 |
|
CRP, mg/dL |
0.9 (0.4–3.3) |
1.8 (0.6–3.7) |
0.263 |
|
CD4 cell count, cells/mm3
|
589 (420–941) |
NA |
NA |
|
Severe manifestations of Mpox |
1 (4) |
1 (3) |
> 0.999 |
|
Tecovirimat used |
20 (80) |
20 (57) |
0.095 |
|
Concurrent antibiotics |
5 (20) |
7 (20) |
> 0.999 |
|
Time from admission to resolution of skin lesion, day |
11 (9–14) |
10 (7–12) |
0.541 |
DISCUSSION
All the patients were male except for two, and transmission was mostly through sexual intercourse or close contact. Similar to the 2022 outbreak,
1 a large proportion of the patients were young men who had sex with men. For the two women, one was infected through needle stick injury and one by sexual contact with a man.
Most patients had no underlying disease other than HIV. In total, 30% (18/60) of patients had already been diagnosed with HIV, and of the 42 patients who had not been diagnosed with HIV before admission, seven (17%) were newly diagnosed with HIV. Syphilis was identified in 8% (4/50) of patients, and other concomitant STIs were identified in 24% (12/51) of patients, similar to previous studies.
11 Patients with Mpox require screening for HIV and other STIs because co-infection with HIV and other STIs is common.
12
Most patients initially presented for constitutional symptoms, and lesions of the anogenital area or other mucocutaneous lesions were each identified in about half of patients. At the time of initial diagnosis, all patients exhibited skin lesions, with the most prevalent presentation being vesiculopustular lesions (82%), followed by maculopapular lesions (60%), and eschars (22%). For the majority of patients, the progress of these skin lesions followed a sequential pattern from maculopapular eruptions to vesicles, pustules, and ultimately crust formation, as depicted in
Fig. 1. The most common site of a rash was the anogenital area, followed by the trunk or limbs, face, and palms or soles, similar to previous reports.
13 In addition, skin lesions appeared only locally in the anogenital area during hospitalization in 5% (3/60) of patients; therefore, caution should be taken with atypical cases, such as those with only local skin lesions, because the diagnosis may be missed.
14
Fig. 1
Temporal changes in typical skin lesions. (A) Maculopapular rash. (B) Vesiculopustular. (C) Crust-forming.
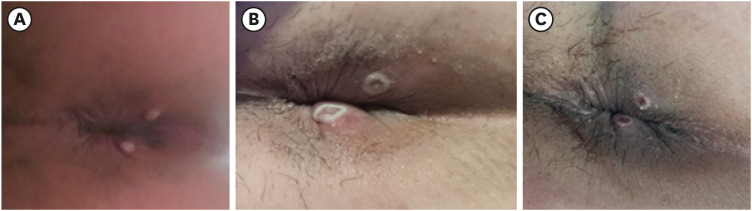
Lymphadenopathy was present in 15% (9/60) of patients, which was a lower rate than in previous reports.
15 This may be due to limitations of this retrospective study, and the inguinal area was affected in all these patients. In addition, there was one case of suspected conjunctivitis, three cases of proctitis, and no case of lung infiltration.
PCR results of skin lesions were mostly positive, but oropharyngeal swabs were positive in about half of patients, and blood was positive in only four patients. Similar to previous studies,
1116 the positivity rate of blood PCR tests was low and no patient was only blood PCR-positive; therefore, it may be sufficient to use only skin and oropharyngeal swabs without collecting blood to diagnose Mpox. A PCR assay was performed on blood in only one out of two patients with severe Mpox, and was positive. Although it cannot be determined in this study due to limited sample size, it is worth investigating in the future whether blood PCR positivity influences severity.
Laboratory abnormalities were not evident and the duration of fever was short. The proportion of patients who received Mpox specific treatment was higher than in previous studies.
11 More patients with HIV received tecovirimat, and the recovery period after hospitalization was longer in tecovirimat-treated patients, but neither was statistically significant.
Seven patients were newly diagnosed with HIV-1 infection among 42 individuals with a previously unknown or negative HIV status (16.7%). This implies that the proportion of undiagnosed people living with HIV-1 in South Korea is relatively high among high-risk STI groups, considering that newly-diagnosed HIV-1 cases were relatively rare in previously reported Mpox case series.
1117
Clinical outcomes were favorable, with only two severe cases and no deaths. The clinical characteristics of patients with and without HIV did not significantly differ.
Infection control during hospitalization followed Korean national guidelines.
18 During treatment, healthcare workers followed standard precautions, contact precautions, and droplet precautions. They wore face shields or goggles, KF94 or higher respirators, non-sterile gloves, and isolation gowns for their personal protective equipment. Discontinuation of isolation was at the discretion of the clinician when no new skin and mucosal lesions developed for 48 hours, mucosal lesions disappeared, and all skin lesions were crusted.
Our study has several limitations. First, this is a retrospective study. Due to the nature of retrospective studies, detailed information such as the number of skin lesions was lacking. Second, this study was conducted at four hospitals and therefore is insufficient to represent South Korea as a whole. However, a large proportion (65.9%) of the Mpox cases reported in South Korea during the study period was included. Third, the number of patients was too small to analyze the effects of treatment or vaccination.
In conclusion, this is the largest study describing the clinical features of Mpox patients in South Korea. A huge proportion of the patients were young men who had sex with men and all patients had skin manifestations. Screening for HIV and other STIs is warranted, and caution is needed for patients with only localized skin lesions. Clinical outcomes were favorable, with only two severe cases and no deaths reported.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download