INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) is a life-saving treatment method for patients with significant cardiorespiratory impairment refractory to conventional therapeutic interventions. Since the inception of the Extracorporeal Life Support Organization (ELSO) in 1989, the use of ECMO has rapidly increased, and the survival rate has steadily improved.
123 According to the latest ELSO report in 2022, the overall survival rate among ECMO recipients is as high as 73% and 61% in neonates and pediatrics, respectively, surpassing the 58% survival rate in adults.
1 With the advancements in experience and equipment, the use of ECMO in critically ill children has increased globally, leading to improved outcomes and an expanded role in pediatric critical care.
3
However, the application of ECMO is more complicated in pediatric patients than in adults. For instance, catheterizing for venovenous (VV) ECMO, which is easily performed using a peripheral approach in adults, can be challenging or even impossible in neonates and young children because of their relatively smaller vessels.
4 Moreover, the relatively shallow insertion of a large cannula into a small body during ECMO is associated with a significant risk of dislocation and bleeding.
5 Furthermore, managing anticoagulation in neonates, who possess developing hemostatic systems and vulnerable brain parenchyma, differs greatly from that in adults and presents unique challenges.
6 Due to the potential for complications and technical difficulties, specialized teams are essential for managing pediatric patients receiving ECMO, who may require a higher level of care than adult patients.
4 Therefore, ECMO is typically available in children’s hospitals with expert pediatric personnel and equipment.
To optimize medical resources and achieve better outcomes in patients receiving ECMO, the number of applications, indications, and clinical outcomes of ECMO must be analyzed. However, only a few centers in Korea that provide ECMO to children have participated in the ELSO registry, resulting in gaps in the ELSO annual report. Moreover, no national or multicenter studies have been conducted on pediatric patients who underwent ECMO in Korea. Therefore, to address this gap, we conducted a study to determine the current status of pediatric ECMO, focusing on its volume and changes in the survival rate over 10 years according to diagnostic indications.
METHODS
This multicenter retrospective study included pediatric patients who received ECMO at 14 hospitals in Korea. We reviewed the records of all patients who received ECMO for any reason from January 2012 to December 2021.
Data collection
Data were collected from the medical records of pediatric patients receiving ECMO aged < 18 years. A patient who underwent ECMO during different hospitalizations was considered a different patient. Data from patients transferred to other centers with ECMO were integrated into one ECMO run and considered for one patient. Demographic, comorbidities, and clinical data were collected. Immunocompromised patients comprised those with hematological or solid organ malignancies, transplants, autoimmune disorders, and primary immune deficiencies. Patients on neonatal ECMO were aged ≤ 28 days during cannulation.
Four diagnostic categories for ECMO were constructed as follows: neonatal respiratory, pediatric respiratory, post-cardiotomy, and cardiac-medical categories. The neonatal respiratory category included neonates treated with ECMO for respiratory failure. Patients of all ages, including neonates, who received ECMO after cardiac surgery, primarily for congenital heart defects, were included in the post-cardiotomy category, which comprised preoperative stabilization, weaning failure from cardiopulmonary bypass, postoperative low cardiac output syndrome, and cardiopulmonary arrest. We categorized patients undergoing post-cardiotomy ECMO into two based on surgical procedures as follows: biventricular palliation/repair and univentricular palliation/repair categories. To enhance this categorization, we employed the Society of Thoracic Surgeons-European Association of Cardio-Thoracic Surgery Congenital Heart Surgery (STAT) Mortality categories.
7 The cardiac-medical ECMO group included cases of heart failure other than post-cardiotomy, such as acute myocarditis, cardiomyopathy, and sudden cardiac arrest with an unknown cause. Pulmonary hypertension and sepsis were also included in this category. The pediatric respiratory ECMO group included children who required ECMO for respiratory support due to various causes of respiratory failure, which were classified according to the patient’s immune status.
The need for extracorporeal cardiopulmonary resuscitation (ECPR) was considered as an additional condition, defined as a case where ECMO flow was established during cardiopulmonary resuscitation (which involves chest compressions or defibrillation) or return of spontaneous circulation was sustained for < 20 minutes.
8
Information on ECMO mode was collected and categorized into two types as follows: VV and venoarterial (VA). Venopulmonary and venoarteriovenous cases were classified as VV and VA, respectively. The central approach involved placing a drain and/or return lines through sternotomy, whereas the peripheral approach involved accessing the neck or femoral vessels. In cases where the mode or approach needed adjustment, we configured the final mode or approach to match the last well-functioning and stable ECMO settings. The transition date from ECMO to a ventricular assist device was recorded as the end date for each patient. For patients with multiple instances, we calculated the total ECMO duration by summing the days since the first application.
The outcome variables included survival to discharge and successful weaning from ECMO with expected recovery, indicating survival within 48 hours after weaning. In this study, the survival rate refers to the proportion of patients who survived until they were discharged from the hospital. To evaluate the trends in these outcomes, we compared periods 1 (2012–2016) and 2 (2017–2021). The main causes of death among non-survivors were classified into four categories: incurable primary diseases requiring ECMO; complications, such as cerebral hemorrhage, other major hemorrhages, cerebral infarction, and sepsis; brain edema and hypoxic brain damage complicated by pre-ECMO conditions; and cause of death unrelated to ECMO support.
Additionally, we categorized the study population by age into neonates and pediatrics to compare survival rates with those in the ELSO registry
1 and subsequently categorized the cases into respiratory, cardiac, and ECPR.
Statistical analysis
Statistical analyses were performed using IBM Statistics for Windows, version 28 (IBM Corp., Armonk, NY, USA). The distribution of continuous variables was described using the median and interquartile range (IQR). For comparison of between-group differences, we used the χ2 and Fisher’s exact tests. Logistic regression was employed to examine mortality-associated factors. P value < 0.05 was considered statistically significant.
Ethics statement
This study was approved by the Institutional Review Board of Pusan National University Yangsan Hospital (04-2022-004) and the local institutional review boards of all participating centers. The requirement of informed consent was waived due to the study’s retrospective nature.
RESULTS
Characteristics of centers
Among the 14 centers involved in this study, two had > 20 ECMO runs per year, three had 10–20 runs, three had 5–10 runs, and six had < 5 runs. Neonatal ECMO and post-cardiotomy ECMO were performed at 11 and 12 centers, respectively.
Overall trends and survival rates
A total of 1,065 ECMO runs were performed in 1,032 patients over the past 10 years.
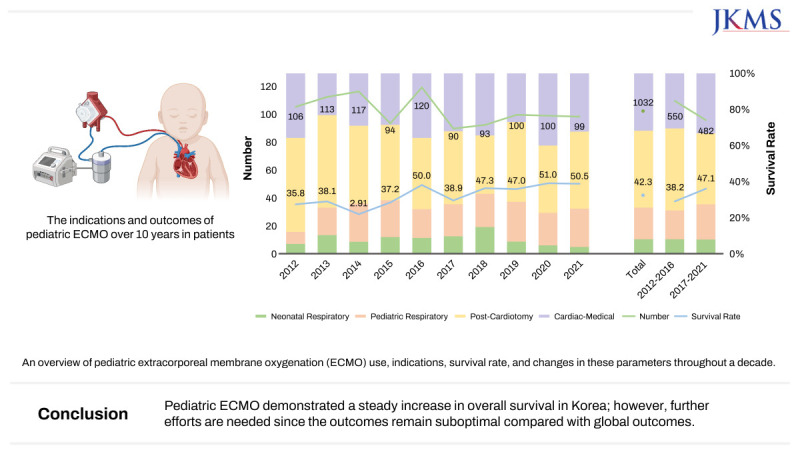
Fig. 1 and
Table 1 demonstrate the total number of patients on ECMO, the proportion of diagnostic categories, the change in survival rate every year, and the comparisons between the two periods. The annual number of ECMO cases remained consistent throughout the 10 years at a range of 90–120 cases. In period 1, 574 ECMO runs were conducted in 550 patients, whereas 491 ECMO runs were performed in 482 patients in period 2.
Fig. 1
The graph presents an overview of pediatric ECMO use, indications, survival rate, and changes in these parameters over 10 years. Among 1032 ECMO cases, the annual numbers remained relatively stable, varying from 90 to 120 cases. Pediatric ECMO was primarily used in post-cardiotomy cases (42.4%), followed by cardiac-medical (31.8%), pediatric respiratory (17.5%), and neonatal respiratory (8.2%) cases. Between periods 1 and 2, the proportion of pediatric respiratory ECMO increased, and that of post-cardiotomy ECMO decreased, while other conditions showed minor changes. During the 10 years, the survival rate showed gradual improvement, with a significant increase from 38.2% to 47.1% between the two 5-year periods.
ECMO = extracorporeal membrane oxygenation.
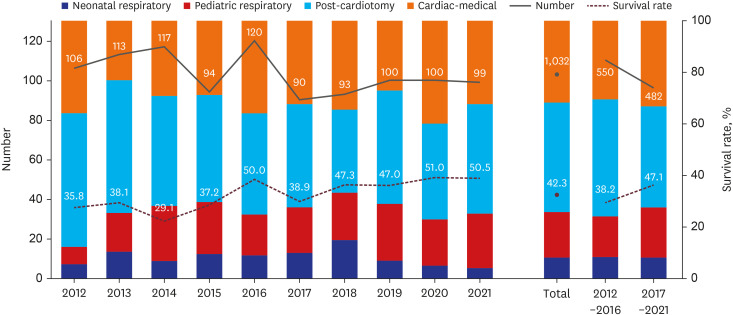
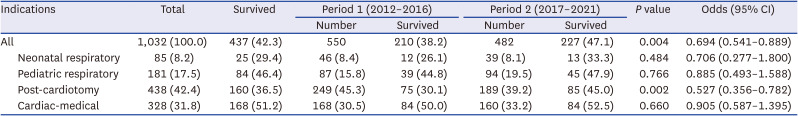
Table 1
Comparison of survival rates between the two 5-year periods according to extracorporeal membrane oxygenation indications
|
Indications |
Total |
Survived |
Period 1 (2012–2016) |
Period 2 (2017–2021) |
P value |
Odds (95% CI) |
|
Number |
Survived |
Number |
Survived |
|
All |
1,032 (100.0) |
437 (42.3) |
550 |
210 (38.2) |
482 |
227 (47.1) |
0.004 |
0.694 (0.541–0.889) |
|
Neonatal respiratory |
85 (8.2) |
25 (29.4) |
46 (8.4) |
12 (26.1) |
39 (8.1) |
13 (33.3) |
0.484 |
0.706 (0.277–1.800) |
|
Pediatric respiratory |
181 (17.5) |
84 (46.4) |
87 (15.8) |
39 (44.8) |
94 (19.5) |
45 (47.9) |
0.766 |
0.885 (0.493–1.588) |
|
Post-cardiotomy |
438 (42.4) |
160 (36.5) |
249 (45.3) |
75 (30.1) |
189 (39.2) |
85 (45.0) |
0.002 |
0.527 (0.356–0.782) |
|
Cardiac-medical |
328 (31.8) |
168 (51.2) |
168 (30.5) |
84 (50.0) |
160 (33.2) |
84 (52.5) |
0.660 |
0.905 (0.587–1.395) |
Pediatric ECMO was most frequently used for post-cardiotomy cases (42.4%), followed by cardiac-medical (31.8%), pediatric respiratory (17.5%), and neonatal respiratory (8.2%) cases. Between periods 1 and 2, the proportion of pediatric respiratory ECMO increased from 15.8% to 19.5%, and that of post-cardiotomy ECMO decreased from 45.3% to 39.2%. Cardiac-medical ECMO increased by 2.6%, whereas neonatal respiratory ECMO decreased by 0.3%.
Over 10 years, the survival rate was 42.3%, indicating a steady increase, with an improvement noted between the two 5-year periods from 38.2% to 47.1%. Among the four groups, the cardiac-medical group had the highest survival rate (51.2%), followed by the pediatric respiratory (46.4%), post-cardiotomy (36.5%), and neonatal respiratory (29.4%) groups. Notable improvements were also noted in survival for post-cardiotomy ECMO between periods 1 and 2 (P = 0.002).
A simple comparison of our 10-year outcomes with those of the cumulative cases enrolled in the ELSO registry through 2021
1 revealed a notable discrepancy in the indications and survival (
Fig. 2). Our study showed a significantly lower proportion of neonatal ECMO utilization (29% vs. 59%) (
Fig. 2A). This contrast can be attributed to higher utilization of pediatric cardiac ECMO (36% vs. 19%) and lower usage of neonatal respiratory ECMO (7% vs. 43%) in our study than that in the ELSO data.
Fig. 2
A simple comparison between data from this study and those from the cumulative cases enrolled until 2021 in the ELSO registry. (A) This study found a lower proportion of neonatal ECMO cases (29%) than that in the ELSO data (59%). The proportion of pediatric cardiac ECMO was considerably higher, while neonatal respiratory ECMO was markedly lower. (B) Lower survival rates (42%) were observed in this study for all categories than those in the ELSO data (61%), except for pediatric cardiac ECMO.
ECMO = extracorporeal membrane oxygenation, ECPR = extracorporeal cardiopulmonary resuscitation, ELSO = Extracorporeal Life Support Organization.
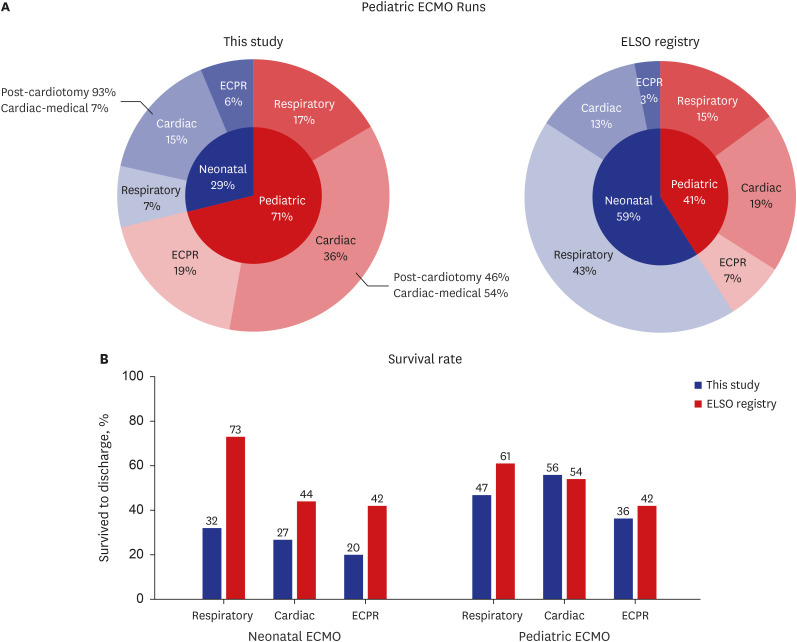
The survival rates were considerably lower across all categories (42% vs. 61%), except in pediatric cardiac ECMO (
Fig. 2B). Additionally, the proportion of ECPR cases appeared higher and had lower survival rates than that in the ELSO registry.
Outcomes
Among 595 non-survivors, 71.1% (n = 423) of the patients died due to incurable primary disease. Another 44 patients (7.4%) succumbed to intracranial hemorrhage, and 40 (6.7%) died of hypoxic brain injury or cerebral edema complicated by pre-ECMO condition. Furthermore, 55 patients (9.2%) died due to other life-threatening complications associated with ECMO. Among these, 34, 15, and 6 patients died due to massive pulmonary or other hemorrhage, sepsis, and cerebral infarction, respectively. The remaining 33 patients (5.5%) died of complications unrelated to ECMO. Neonates had higher rates of cerebral hemorrhage than older children (14.4% vs. 5.9%) (P < 0.001).
Among all patients, 561 (53.3%) were successfully weaned from ECMO, whereas 139 (24.8%) did not survive after weaning. Of patients who died after successful weaning, 63.3% died of incurable primary diseases. Furthermore, excluding the 20.2% of deaths unrelated to ECMO, the median time until death was 18 days (IQR: 9–41).
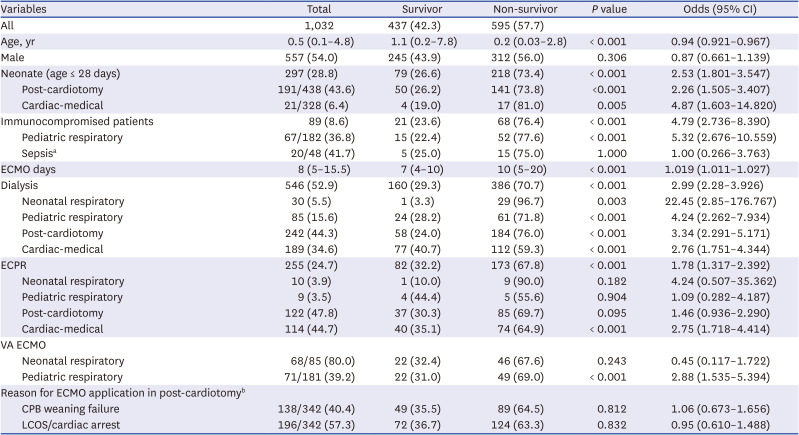
Table 2 shows the comparisons of the clinical characteristics of the survivors and non-survivors and the results of logistic regression analysis for mortality. The median age in the overall patient population was 0.5 years, with survivors being significantly older than non-survivors (
P < 0.001). The median duration of ECMO was 8 days, with survivors having a shorter duration than non-survivors (
P < 0.001). Neonates had a significantly higher mortality risk in post-cardiotomy (
P < 0.001) and cardiac-medical (
P = 0.005) ECMO than older children.
Table 2
Clinical characteristics and logistic regression analysis for mortality in survivors vs. non-survivors
|
Variables |
Total |
Survivor |
Non-survivor |
P value |
Odds (95% CI) |
|
All |
1,032 |
437 (42.3) |
595 (57.7) |
|
|
|
Age, yr |
0.5 (0.1–4.8) |
1.1 (0.2–7.8) |
0.2 (0.03–2.8) |
< 0.001 |
0.94 (0.921–0.967) |
|
Male |
557 (54.0) |
245 (43.9) |
312 (56.0) |
0.306 |
0.87 (0.661–1.139) |
|
Neonate (age ≤ 28 days) |
297 (28.8) |
79 (26.6) |
218 (73.4) |
< 0.001 |
2.53 (1.801–3.547) |
|
Post-cardiotomy |
191/438 (43.6) |
50 (26.2) |
141 (73.8) |
<0.001 |
2.26 (1.505–3.407) |
|
Cardiac-medical |
21/328 (6.4) |
4 (19.0) |
17 (81.0) |
0.005 |
4.87 (1.603–14.820) |
|
Immunocompromised patients |
89 (8.6) |
21 (23.6) |
68 (76.4) |
< 0.001 |
4.79 (2.736–8.390) |
|
Pediatric respiratory |
67/182 (36.8) |
15 (22.4) |
52 (77.6) |
< 0.001 |
5.32 (2.676–10.559) |
|
Sepsisa
|
20/48 (41.7) |
5 (25.0) |
15 (75.0) |
1.000 |
1.00 (0.266–3.763) |
|
ECMO days |
8 (5–15.5) |
7 (4–10) |
10 (5–20) |
< 0.001 |
1.019 (1.011–1.027) |
|
Dialysis |
546 (52.9) |
160 (29.3) |
386 (70.7) |
< 0.001 |
2.99 (2.28–3.926) |
|
Neonatal respiratory |
30 (5.5) |
1 (3.3) |
29 (96.7) |
0.003 |
22.45 (2.85–176.767) |
|
Pediatric respiratory |
85 (15.6) |
24 (28.2) |
61 (71.8) |
< 0.001 |
4.24 (2.262–7.934) |
|
Post-cardiotomy |
242 (44.3) |
58 (24.0) |
184 (76.0) |
< 0.001 |
3.34 (2.291–5.171) |
|
Cardiac-medical |
189 (34.6) |
77 (40.7) |
112 (59.3) |
< 0.001 |
2.76 (1.751–4.344) |
|
ECPR |
255 (24.7) |
82 (32.2) |
173 (67.8) |
< 0.001 |
1.78 (1.317–2.392) |
|
Neonatal respiratory |
10 (3.9) |
1 (10.0) |
9 (90.0) |
0.182 |
4.24 (0.507–35.362) |
|
Pediatric respiratory |
9 (3.5) |
4 (44.4) |
5 (55.6) |
0.904 |
1.09 (0.282–4.187) |
|
Post-cardiotomy |
122 (47.8) |
37 (30.3) |
85 (69.7) |
0.095 |
1.46 (0.936–2.290) |
|
Cardiac-medical |
114 (44.7) |
40 (35.1) |
74 (64.9) |
< 0.001 |
2.75 (1.718–4.414) |
|
VA ECMO |
|
|
|
|
|
|
Neonatal respiratory |
68/85 (80.0) |
22 (32.4) |
46 (67.6) |
0.243 |
0.45 (0.117–1.722) |
|
Pediatric respiratory |
71/181 (39.2) |
22 (31.0) |
49 (69.0) |
< 0.001 |
2.88 (1.535–5.394) |
|
Reason for ECMO application in post-cardiotomyb
|
|
|
|
|
|
|
CPB weaning failure |
138/342 (40.4) |
49 (35.5) |
89 (64.5) |
0.812 |
1.06 (0.673–1.656) |
|
LCOS/cardiac arrest |
196/342 (57.3) |
72 (36.7) |
124 (63.3) |
0.832 |
0.95 (0.610–1.488) |
Dialysis was performed in 52.9% (n = 546) patients, most of whom received continuous kidney replacement therapy (n = 408, 74.7%). Acute kidney injury (AKI) requiring dialysis was most common in the cardiac-medical ECMO category, particularly in patients with sepsis (83.3%). Patients requiring dialysis for AKI had a significant mortality risk across all diagnostic categories (P < 0.05).
In total, 255 ECPR runs were performed for 10 years, with almost all cases involving cardiac ECMO, including post-cardiotomy (47.8%) and cardiac-medical (44.7%) ECMO (
Table 2). Non-survivors had a significantly higher implementation of ECPR than survivors, particularly in cardiac-medical ECMO (
P < 0.001). The overall survival rate of patients who underwent ECPR was 32.2%, which increased from 27.1% (38/140) in period 1 to 38.3% (44/115) in period 2, without a significant difference (
P = 0.080).
During the study period, the smallest patient who received ECMO weighed 1.04 kg with Tetralogy of Fallot (TOF) and died. The smallest surviving patient weighed 1.4 kg with total anomalous pulmonary venous return.
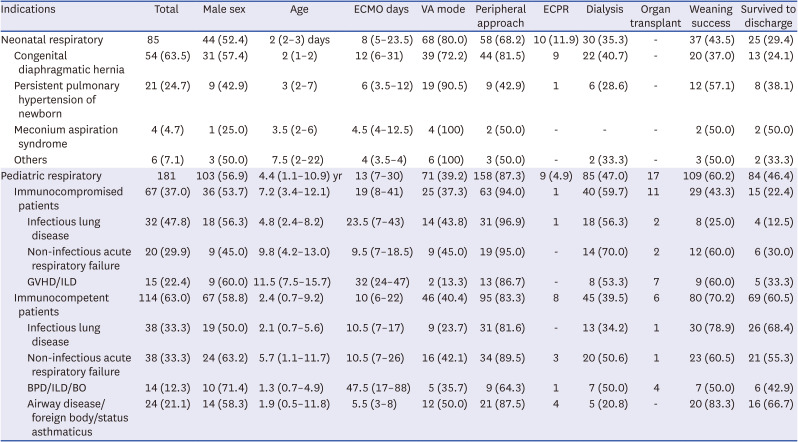
Neonatal respiratory ECMO
The most common neonatal respiratory diagnosis that required ECMO was congenital diaphragmatic hernia (CDH) (63.5%), followed by persistent pulmonary hypertension (PPHN) (24.7%) (
Table 3). The median age at which ECMO was initiated was 2 days, and the median duration of ECMO runs was 8 days.
Table 3
Clinical characteristics of neonatal and pediatric respiratory ECMO cases
|
Indications |
Total |
Male sex |
Age |
ECMO days |
VA mode |
Peripheral approach |
ECPR |
Dialysis |
Organ transplant |
Weaning success |
Survived to discharge |
|
Neonatal respiratory |
85 |
44 (52.4) |
2 (2–3) days |
8 (5–23.5) |
68 (80.0) |
58 (68.2) |
10 (11.9) |
30 (35.3) |
- |
37 (43.5) |
25 (29.4) |
|
Congenital diaphragmatic hernia |
54 (63.5) |
31 (57.4) |
2 (1–2) |
12 (6–31) |
39 (72.2) |
44 (81.5) |
9 |
22 (40.7) |
- |
20 (37.0) |
13 (24.1) |
|
Persistent pulmonary hypertension of newborn |
21 (24.7) |
9 (42.9) |
3 (2–7) |
6 (3.5–12) |
19 (90.5) |
9 (42.9) |
1 |
6 (28.6) |
- |
12 (57.1) |
8 (38.1) |
|
Meconium aspiration syndrome |
4 (4.7) |
1 (25.0) |
3.5 (2–6) |
4.5 (4–12.5) |
4 (100) |
2 (50.0) |
- |
- |
- |
2 (50.0) |
2 (50.0) |
|
Others |
6 (7.1) |
3 (50.0) |
7.5 (2–22) |
4 (3.5–4) |
6 (100) |
3 (50.0) |
- |
2 (33.3) |
- |
3 (50.0) |
2 (33.3) |
|
Pediatric respiratory |
181 |
103 (56.9) |
4.4 (1.1–10.9) yr |
13 (7–30) |
71 (39.2) |
158 (87.3) |
9 (4.9) |
85 (47.0) |
17 |
109 (60.2) |
84 (46.4) |
|
Immunocompromised patients |
67 (37.0) |
36 (53.7) |
7.2 (3.4–12.1) |
19 (8–41) |
25 (37.3) |
63 (94.0) |
1 |
40 (59.7) |
11 |
29 (43.3) |
15 (22.4) |
|
|
Infectious lung disease |
32 (47.8) |
18 (56.3) |
4.8 (2.4–8.2) |
23.5 (7–43) |
14 (43.8) |
31 (96.9) |
1 |
18 (56.3) |
2 |
8 (25.0) |
4 (12.5) |
|
|
Non-infectious acute respiratory failure |
20 (29.9) |
9 (45.0) |
9.8 (4.2–13.0) |
9.5 (7–18.5) |
9 (45.0) |
19 (95.0) |
- |
14 (70.0) |
2 |
12 (60.0) |
6 (30.0) |
|
|
GVHD/ILD |
15 (22.4) |
9 (60.0) |
11.5 (7.5–15.7) |
32 (24–47) |
2 (13.3) |
13 (86.7) |
- |
8 (53.3) |
7 |
9 (60.0) |
5 (33.3) |
|
Immunocompetent patients |
114 (63.0) |
67 (58.8) |
2.4 (0.7–9.2) |
10 (6–22) |
46 (40.4) |
95 (83.3) |
8 |
45 (39.5) |
6 |
80 (70.2) |
69 (60.5) |
|
|
Infectious lung disease |
38 (33.3) |
19 (50.0) |
2.1 (0.7–5.6) |
10.5 (7–17) |
9 (23.7) |
31 (81.6) |
- |
13 (34.2) |
1 |
30 (78.9) |
26 (68.4) |
|
|
Non-infectious acute respiratory failure |
38 (33.3) |
24 (63.2) |
5.7 (1.1–11.7) |
10.5 (7–26) |
16 (42.1) |
34 (89.5) |
3 |
20 (50.6) |
1 |
23 (60.5) |
21 (55.3) |
|
|
BPD/ILD/BO |
14 (12.3) |
10 (71.4) |
1.3 (0.7–4.9) |
47.5 (17–88) |
5 (35.7) |
9 (64.3) |
1 |
7 (50.0) |
4 |
7 (50.0) |
6 (42.9) |
|
|
Airway disease/foreign body/status asthmaticus |
24 (21.1) |
14 (58.3) |
1.9 (0.5–11.8) |
5.5 (3–8) |
12 (50.0) |
21 (87.5) |
4 |
5 (20.8) |
- |
20 (83.3) |
16 (66.7) |
The survival rate in this group was 29.4%, which increased from period 1 (26.1%) to period 2 (33.3%) (P = 0.484). Among patients who required ECMO, those with CDH had the lowest survival rate (24.1%), whereas those with meconium aspiration syndrome (MAS) had 50%. Neonates with CDH experienced an increase in the survival rate from period 1 (10.3%) to period 2 (40%) (odds ratio, 0.2; 95% confidence interval, 0.04–0.73; P = 0.023).
VA cannulation (80.0%) and the peripheral approach (68.2%) were preferred over VV cannulation and the central approach for neonatal respiratory ECMO. No significant difference was found in survival when comparing VA cannulation with VV cannulation (
Table 2).
Pediatric respiratory ECMO
In pediatric respiratory ECMO cases, 37% of the patients were immunocompromised (
Table 3). The predominant condition was infectious lung disease regardless of the patient’s immune status. The median age of patients undergoing pediatric respiratory ECMO was 4.4 years. Depending on the indication, the duration of ECMO runs ranged from 1 to 139 days, with a median duration of 13 days.
The overall survival rate was 46.4%, increasing from 44.8% to 47.9% between the two periods (
P = 0.766). Notably, the survival rates varied significantly depending on immune status. Immunocompromised patients had lower survival rates with infectious diseases than immunocompetent patients. Additionally, immunocompromised status was associated with a significantly higher risk of mortality (
Table 2). In this group, VV ECMO (60.8%) and peripheral ECMO line insertion (87.3%) were the preferred options. However, younger patients with respiratory failure were more likely to receive VA ECMO (2.4 vs. 5.4 years,
P < 0.001) and central approach ECMO line insertion (1.0 vs. 5.2 years,
P < 0.001). Furthermore, using VA ECMO significantly increased mortality in this group by approximately 2.9-fold compared to VV ECMO (
P < 0.001) (
Table 2).
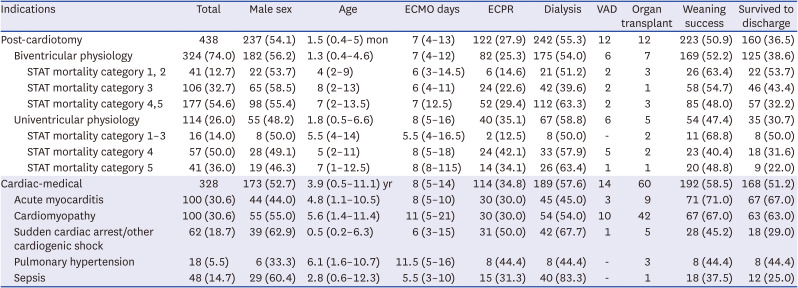
Post-cardiotomy ECMO
In the post-cardiotomy ECMO category, 324 and 114 patients were in the biventricular and univentricular physiology groups, respectively (
Table 4). The median age at ECMO initiation was 1.5 months, and among these patients, 191 (43.6%) were neonates.
Fig. 2A shows that 93% of neonatal cardiac ECMO cases were post-cardiotomy cases. Neonates underwent surgeries in a higher STAT mortality category than children (
P < 0.001).
Table 4
Clinical characteristics of Post-cardiotomy and Cardiac-medical ECMO cases
|
Indications |
Total |
Male sex |
Age |
ECMO days |
ECPR |
Dialysis |
VAD |
Organ transplant |
Weaning success |
Survived to discharge |
|
Post-cardiotomy |
438 |
237 (54.1) |
1.5 (0.4–5) mon |
7 (4–13) |
122 (27.9) |
242 (55.3) |
12 |
12 |
223 (50.9) |
160 (36.5) |
|
Biventricular physiology |
324 (74.0) |
182 (56.2) |
1.3 (0.4–4.6) |
7 (4–12) |
82 (25.3) |
175 (54.0) |
6 |
7 |
169 (52.2) |
125 (38.6) |
|
|
STAT mortality category 1, 2 |
41 (12.7) |
22 (53.7) |
4 (2–9) |
6 (3–14.5) |
6 (14.6) |
21 (51.2) |
2 |
3 |
26 (63.4) |
22 (53.7) |
|
|
STAT mortality category 3 |
106 (32.7) |
65 (58.5) |
8 (2–13) |
6 (4–11) |
24 (22.6) |
42 (39.6) |
2 |
1 |
58 (54.7) |
46 (43.4) |
|
|
STAT mortality category 4,5 |
177 (54.6) |
98 (55.4) |
7 (2–13.5) |
7 (12.5) |
52 (29.4) |
112 (63.3) |
2 |
3 |
85 (48.0) |
57 (32.2) |
|
Univentricular physiology |
114 (26.0) |
55 (48.2) |
1.8 (0.5–6.6) |
8 (5–16) |
40 (35.1) |
67 (58.8) |
6 |
5 |
54 (47.4) |
35 (30.7) |
|
|
STAT mortality category 1–3 |
16 (14.0) |
8 (50.0) |
5.5 (4–14) |
5.5 (4–16.5) |
2 (12.5) |
8 (50.0) |
- |
2 |
11 (68.8) |
8 (50.0) |
|
|
STAT mortality category 4 |
57 (50.0) |
28 (49.1) |
5 (2–11) |
8 (5–18) |
24 (42.1) |
33 (57.9) |
5 |
2 |
23 (40.4) |
18 (31.6) |
|
|
STAT mortality category 5 |
41 (36.0) |
19 (46.3) |
7 (1–12.5) |
8 (8–115) |
14 (34.1) |
26 (63.4) |
1 |
1 |
20 (48.8) |
9 (22.0) |
|
Cardiac-medical |
328 |
173 (52.7) |
3.9 (0.5–11.1) yr |
8 (5–14) |
114 (34.8) |
189 (57.6) |
14 |
60 |
192 (58.5) |
168 (51.2) |
|
Acute myocarditis |
100 (30.6) |
44 (44.0) |
4.8 (1.1–10.5) |
8 (5–10) |
30 (30.0) |
45 (45.0) |
3 |
9 |
71 (71.0) |
67 (67.0) |
|
Cardiomyopathy |
100 (30.6) |
55 (55.0) |
5.6 (1.4–11.4) |
11 (5–21) |
30 (30.0) |
54 (54.0) |
10 |
42 |
67 (67.0) |
63 (63.0) |
|
Sudden cardiac arrest/other cardiogenic shock |
62 (18.7) |
39 (62.9) |
0.5 (0.2–6.3) |
6 (3–15) |
31 (50.0) |
42 (67.7) |
1 |
5 |
28 (45.2) |
18 (29.0) |
|
Pulmonary hypertension |
18 (5.5) |
6 (33.3) |
6.1 (1.6–10.7) |
11.5 (5–16) |
8 (44.4) |
8 (44.4) |
- |
3 |
8 (44.4) |
8 (44.4) |
|
Sepsis |
48 (14.7) |
29 (60.4) |
2.8 (0.6–12.3) |
5.5 (3–10) |
15 (31.3) |
40 (83.3) |
- |
1 |
18 (37.5) |
12 (25.0) |
The overall survival rate was 36.5%. The univentricular group exhibited a generally higher STAT category and a lower survival rate than the biventricular group. Remarkably, the survival rate of patients in the post-cardiotomy ECMO group improved from 30.1% in period 1 to 45.0% in period 2 (
P = 0.002). The reasons for ECMO use in 342 available cases among the post-cardiotomy group were as follows: 138 (40.4%), 145 (42.4%), 51 (14.9%), and 8 (2.3%) cases due to weaning failure from cardiopulmonary bypass, for postoperative low cardiac output syndrome, following cardiopulmonary arrest, and for preoperative stabilization, respectively. No statistically significant association was observed between the reason for ECMO application and patient survival (
Table 2).
Cardiac-medical ECMO
Acute myocarditis (30.6%) and cardiomyopathy (30.6%) were the most common etiologies in the cardiac-medial ECMO group (
Table 4). The median age of these patients was 3.9 years, and the median duration of ECMO was 8 days.
With an overall survival rate of 51.2%, this was the highest among all four diagnostic categories. Particularly, the survival rate of acute myocarditis and cardiomyopathy reached 67% and 63%, respectively. Survival improved from 50% to 52.5% between the two periods without statistical significance. Patients with sepsis had the lowest survival rate (25.0%) in cardiac-medical ECMO cases with 41.7% having immunocompromised conditions. However, no significant relationship was found between patient immune status and mortality (
Table 2).
DISCUSSION
To the best of our knowledge, this retrospective multicenter study provides the first comprehensive evaluation of pediatric ECMO in Korea. In 1991, Choi et al.
9 documented the first four cases of ECMO in Korea, including that of a 2-year-old male patient who developed respiratory failure after surgery for TOF, marking Korea’s first survival case. Since then, ECMO usage in Korea has steadily increased.
10 However, the available research primarily focuses on adult cases with limited large-scale multicenter studies on pediatric ECMO. This study involved hospitals that could provide ECMO to pediatric patients, along with Korean centers that perform the therapy on at least five cases yearly. Consequently, the data presented in this study can be considered representative of Korea. It details pediatric ECMO usage and outcomes over the past decade, encompassing vital clinical aspects and comparing them with global data.
The ELSO registry indicates increasing ECMO use in adults and children with cardiac and respiratory failure, except for neonates.
2 Our data showed a 3.7% increase in pediatric respiratory ECMO cases, and these findings align with the global trend of expanding ECMO use in pediatric respiratory failure.
13 Advancements in surgical techniques, anticoagulation strategies, and specialized equipment have substantially improved the accessibility of ECMO therapy to children, regardless of their size or weight. Additionally, these developments have broadened the scope of indications for ECMO and can enhance the overall survival rates of critically ill pediatric patients.
However, we found no significant change in the annual number of pediatric ECMO cases in Korea. These gaps should be re-evaluated, given the decreasing pediatric population in Korea because of the declining birth rate over the past decade.
11 Data from the Korean Statistical Information Service indicates that the number of children aged < 19 years has decreased by approximately 13%, from 5.2 million in period 1 to 4.5 million in period 2 (
Supplementary Table 1).
12 Based on our study, the number of ECMO cases per million cases has increased slightly, from 10.4 to 10.9. This implies that the number of pediatric patients receiving ECMO in Korea is increasing, which is consistent with global trends. In this context, our study revealed a 6.1% decrease in post-cardiotomy ECMO cases between the two periods, possibly due to the rapidly declining birth rates in Korea
11 and the reduced number of cases with complex congenital heart disease requiring ECMO.
13
Our study reveals a steady increase in patient survival rates over the past decade, with notable improvements observed between the two periods. However, significantly lower survival rates were found in all categories except for pediatric cardiac ECMO than those in the ELSO registry. This discrepancy was particularly pronounced in neonates compared to the pediatric population. Therefore, emphasizing that this difference arises from a notable variation in case distribution is crucial, with a significantly lower number of neonatal ECMO cases primarily applied in cardiac patients.
For neonatal respiratory ECMO cases, our study found a significantly lower survival rate (29.4%), mainly due to CDH (24%). In contrast, the ELSO report revealed higher survival rates for conditions including MAS (93%), respiratory distress syndrome (79%), and PPHN (74%) than for CDH (50%).
3 The proportion of neonatal respiratory ECMO cases was smaller in this study than that in the ELSO data,
1 with CDH (64%) and PPHN (25%) as primary indications, whereas ELSO showed CDH (32%), MAS (24%), and PPHN (21%).
3 This indicates that ECMO is less frequently used in Korea for non-CDH neonatal respiratory failure, leading to lower survival rates than those of global data. Similarly, neonatal cardiac ECMO accounts for most of the post-cardiotomy cases, with lower survival rates than cardiac-medical ECMO. Furthermore, the STAT mortality category among patients in the neonatal post-cardiotomy group tended to be higher than that in children, indicating a greater prevalence of complex and high-risk cardiac anomalies that pose surgical challenges.
Nevertheless, the improvement in survival was noticeable between the two periods in cases where ECMO was used to manage patients with CDH and those receiving post-cardiotomy ECMO. Achievements in the surgical management of congenital heart defect and neonatal care standards in Korea could be responsible for these positive outcomes.
14151617 Moreover, a study conducted at a single center in Korea reported a survival rate of 31.4% for patients with CDH treated with ECMO, which was higher than our results.
18 Several studies have demonstrated that an increase in cases of ECMO implementation within hospitals is associated with better survival rates.
1719202122 Although our study did not compare survival rates based on center volume, accumulation of experience is key in ECMO application.
Furthermore, respiratory ECMO cases in both neonatal and pediatric populations demonstrated lower overall survival rates compared to the ELSO report. As ECMO is often used as a last resort in acute respiratory failure when conventional treatments fail, inaccurate patient stratification and suboptimal treatment, including improper ECMO timing, may contribute to poor outcomes. In our study, about 40% of pediatric respiratory ECMO cases used VA ECMO, which was significantly associated with patient mortality. We believe that VA ECMO was utilized for severe respiratory failure already leading to hemodynamic instability. Due to the lack of universally accepted criteria for pediatric respiratory ECMO initiation, the ELSO emphasizes the importance of timely implementation ECMO to avoid severe hypoxia, end-organ damage, and hemodynamic instability.
23 Recent studies on adults have confirmed that early ECMO has more beneficial outcomes than conventional therapy, especially in severe acute respiratory distress syndrome cases.
242526 Therefore, it is imperative to establish more appropriate indications and consider ECMO early in the treatment process by accurately stratifying patients. This approach is pivotal in enhancing outcomes in neonatal and pediatric respiratory ECMO cases.
To improve the survival rates of pediatric patients receiving ECMO, a multifaceted approach is necessary. This approach includes the development of advanced ECMO strategies, establishing precise indications for ECMO, fostering close communication and collaboration among medical staff involved in ECMO management (comprising neonatologists, pediatric intensivists, pediatric cardiologists, and cardiothoracic surgeons), and the accumulation of technical expertise in ECMO procedures. Additionally, as more pediatric ECMO experience and data accumulate in the future, this approach enables more comprehensive group comparisons and prospective studies with various parameters. These research efforts will provide valuable insights and ultimately enhance patient outcomes.
Additionally, despite technological advances, ECMO outcomes vary depending on the infrastructure of the center providing care,
21272829 particularly when managing a heterogeneous population of pediatric patients receiving ECMO. A 2015 survey reported inadequate manpower and facilities for pediatric intensive care in Korea,
30 resulting in limited space and medical staff for managing the increasing number of pediatric patients undergoing ECMO. Therefore, to improve outcomes in this population, strengthening pediatric intensive care resources in Korea beyond relying solely on technological developments is crucial.
This retrospective study has some limitations. First, potential inaccuracies in clinical information and a risk of bias across researchers and centers may exist. Nonetheless, the impact of information bias is relatively low since the study was based on > 1,000 cases with only minimal clinical information collected. However, these limited data also constrain the study’s capacity for comprehensive analysis and identification of significant risk factors. We identified some factors related to mortality using univariate regression analysis; however, recognizing that analyzing risk with limited data can potentially lead to misinterpretation of causality is crucial. Therefore, future research should include long-term outcomes, risk factor identification, and in-depth prognostic analysis. Second, due to the significant disparity in patient numbers between this study and the ELSO report, making a ‘simple comparison’ could have introduced errors. Nevertheless, the indications and outcomes of the two groups are distinct and divergent. Third, despite potential selection bias from the relatively low number of 14 Korean hospitals, the study data are still representative of the national data since most hospitals that can provide ECMO to children participated in the study. Finally, since the outcomes of ECMO are associated with the accumulated experience of each center, further studies are needed to explore the effects of inter-center differences in indications and clinical practice of ECMO.
In conclusion, our study primarily assessed pediatric ECMO indications and outcomes in Korea over the last decade. This multicenter study on pediatric ECMO in Korea revealed a steady increase in overall survival despite unchanging annual case numbers. However, the significantly low survival rates in Korea compared to global data highlight the need for strategies to improve pediatric ECMO outcomes. Therefore, collaborative initiatives and shared research endeavors among medical centers should be pursued to refine and enhance the development and implementation of pediatric ECMO protocols.










 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download