This article has been
cited by other articles in ScienceCentral.
CASE PRESENTATION
Dr. Kabsoo Shin: A 66-year-old woman visited the clinic with a complaint of dizziness and left side weakness for the past month. Five years ago, she was diagnosed with hormone receptor-negative, HER2-positive breast cancer and underwent a mastectomy with axillary lymph node dissection (pT3, pN3a) at another hospital. After the surgery, she declined adjuvant treatment and was under observation. Nine months later, she experienced a recurrence with pulmonary metastasis. She was treated with palliative first-line treatment comprising docetaxel, trastuzumab and pertuzumab (THP) and subsequent trastuzumab and pertuzumab maintenance. She remained on the first line treatment for 36 months, but showed progression of pulmonary metastasis. Trastuzumab emtansine (T-DM1) was administered as second-line treatment. After six cycles of 3-weekly T-DM1, brain metastases were detected on brain magnetic resonance imaging (MRI). She refused additional anti-cancer treatment, agreeing instead to hospice care. She received symptomatic management for four months. However, she came to our hospital seeking further treatment due to the rapid worsening of her dizziness and left side weakness.
At the time of her visit, she was taking daily 4 to 8 mg of dexamethasone for controlling dizziness and headache. Physical examination was unremarkable. On neurological examination, she was alert but appeared confused and was unable to follow a three-step command. She exhibited left facial palsy and weakness in her left arm and leg (Medical Research Council scale grade III). The finger-to-nose test revealed ataxic movements. She intermittently exhibited aggressive behavior and was uncooperative with medical staff. There were no specific findings in the complete blood count and blood chemistry.
IMAGE PRESENTATION
Dr. Kabsoo Shin: We performed MRI and computed tomography (CT) scans for initial evaluation. May we review the imaging findings?
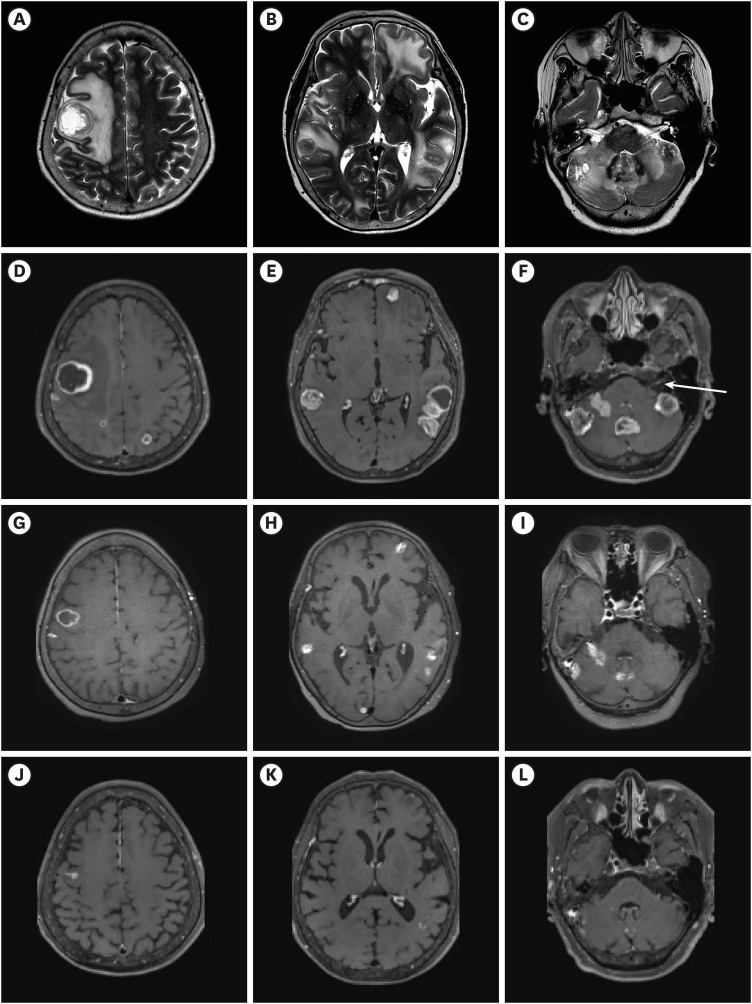
Dr. Ga Eun Park: Contrast-enhanced brain MRI was performed for initial brain evaluation (
Fig. 1A-F). It reveals more than 20 multiple, variably sized nodular and rim-enhancing lesions in the brain (
Fig. 1D-F). A lesion measuring 3.3 cm in the right frontal lobe exhibits remarkable perilesional edema (
Fig. 1A-C). Multiple lesions, up to 3.7 cm in size, are present in the cerebellum (
Fig. 1F). A tiny nodular enhancing lesion is observed in the complex of the seventh and eighth cranial nerves on the left (
Fig. 1F, arrow). These findings are compatible with brain metastases. Additionally, the metastatic lesions seen in the brain correspond to the patient's symptoms of dizziness, left side weakness, and left facial palsy.
Fig. 1
Baseline and follow-up brain magnetic resonance imaging of the patient. (A-C) Axial T2 weighted image of brain MRI before trastuzumab-deruxtecan. The perilesional edema seen around the multiple lesions appears as high signal intensity. (D-L) Axial contrast-enhanced T1 weighted images of brain MRI. (D-F) Multiple, variably sized nodular and rim-enhancing lesions are seen throughout the brain before treatment. A tiny nodular enhancing lesion is observed in the complex of the seventh and eighth cranial nerves on the left (F, arrow). (G-I) After four cycles of trastuzumab-deruxtecan, multiple variable lesions in the brain have significantly decreased. (J-L) After eight cycles of trastuzumab-deruxtecan, most of the brain lesions have regressed, showing a near-complete response.
MRI = magnetic resonance imaging.
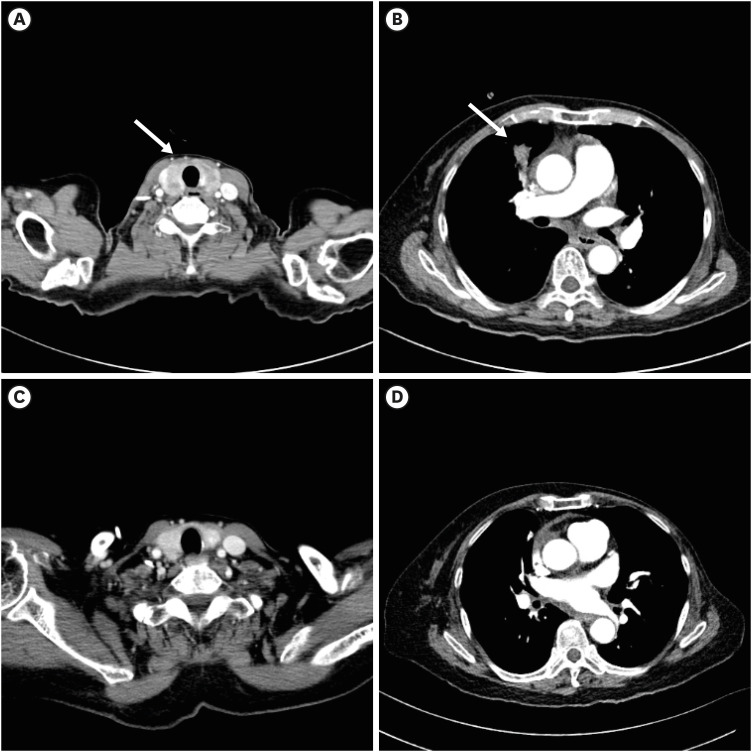
Enhanced neck CT shows diffuse low-attenuating infiltration in the thyroid gland, suggesting thyroid metastases (
Fig. 2A). Enhanced chest CT reveals an irregular nodular lesion in the right middle lobe, suggesting lung metastasis (
Fig. 2B, arrow).
Fig. 2
Baseline and follow-up thoracic computed tomography of the patient. (A, arrow) Enhanced neck CT shows diffuse low-attenuating infiltration in the thyroid gland, suggesting thyroid metastases. (B, arrow) Enhanced chest CT reveals an irregular nodular lesion in the right middle lobe, suggesting lung metastasis. (C, D) After eight cycles of trastuzumab-deruxtecan, most of the lesions have regressed, showing a near-complete response.
CT = computed tomography.
DIAGNOSIS
HER2-positive breast cancer with brain, lung and thyroid metastases.
TREATMENT PLAN AND CLINICAL COURSE
Dr. Kabsoo Shin: A multidisciplinary discussion was held to establish a treatment plan. Whole brain radiation therapy (WBRT) was initially considered, and was expected to provide a rapid response for multiple brain metastases accompanied by severe neurological symptoms such as confused mentality, dizziness, and hemiplegia. However, it was deemed impractical to proceed with WBRT, taking into account the patient's poor performance status and occasional aggressive behavior, which made cooperation difficult. Additionally, progression of the extracranial disease was a concern. Therefore, as an alternative, a systemic treatment expected to provide a rapid and effective intracranial response was decided upon. Accordingly, trastuzumab deruxtecan (T-DXd) was administered at a dose of 5.4 mg/kg every three weeks.
After receiving T-DXd, the patient showed improvement in dizziness, confused mentality, and facial palsy from the next day. On the fourth day after starting T-DXd, a neurologic exam revealed improvement in her left side weakness to grade IV. She was able to walk independently and was discharged with improved neurologic symptoms on the eighth day after T-DXd treatment. A response evaluation conducted after four cycles of T-DXd showed a reduction in the size of multiple brain metastatic lesions on the brain MRI, and neck and chest CT scans also revealed size reductions in extracranial lesions, including lung and thyroid metastatic lesions. (
Figs. 1G-I and
2) Following eight cycles of T-DXd, her left side motor function completely recovered to grade V, and she remained neurologically stable except for mild intermittent dizziness. The brain MRI showed near-complete regression of most lesions (
Fig. 1J-L). During the treatment of T-DXd. she experienced repeated Grade 2 nausea and fatigue, leading to dose reduction, but she continued to receive T-DXd and has completed 11 cycles to date.
GENERAL INTRODUCTION OF THE DISEASE MANAGEMENT
Dr. Kabsoo Shin: Generally, in solid cancers with newly diagnosed or progressing active brain metastases, local treatment is primarily considered.
12 Depending on the number, size, and location, neurosurgery, stereotactic radiotherapy (SRT), stereotactic radiosurgery (SRS), and WBRT can be options. WBRT is spared if possible due to the side effect of neurocognitive decline, and SRS is mainly considered when the number of brain metastases is less than 10 and the size is less than 2 cm (conditionally less than 4 cm).
3 WBRT is considered when SRS or SRT is not indicated. Furthermore, after local treatment, systemic treatment based on the primary tumor and previous treatment should be considered. If local treatment is not feasible or there is accompanying extracranial progression, systemic treatment with central nervous system (CNS) activity could be considered over the local treatment. Additionally, in cases where brain metastases are stable or asymptomatic, and only small lesions are present, systemic treatment can be primarily considered depending on the availability of effective systemic treatment options. The decision on such treatment strategies is crucially dependent on multidisciplinary collaboration and should be individualized based on the primary tumor and the clinical situation of the patient.
DISCUSSION
Dr. Jieun Lee: This case report demonstrates a favorable response of T-DXd in a patient with severe neurologic deterioration and poor performance status due to extensive and active brain metastases of a HER2-positive breast cancer. It is particularly interesting that near-complete regression was observed in both intracranial and extracranial lesions, and that neurological symptoms showed rapid improvement within several days.
Dr. Hye Won Mun: Which subtype of breast cancer commonly shows brain metastases?
Dr. Jieun Lee: In hormone receptor-positive breast cancer, about 5% of patients exhibit brain metastases at the time of diagnosis when the disease is metastatic. The metastatic HER2-positive and triple-negative breast cancer (TNBC) subtypes are more prone to developing brain metastases. In these subtypes, over 10% of patients have brain metastases at the time of metastatic disease diagnosis.
4 Moreover, in metastatic HER2-positive breast cancer, recent advancements in treatments have extended survival times, leading to as many as 50% of patients experiencing brain metastases during the course of their treatment. A comparable proportion of patients with metastatic TNBC also develop brain metastasis during treatment.
56 Consequently, these subtypes necessitate more vigilant monitoring for neurological symptoms, and a proactive approach to radiological evaluation, such as brain MRI, is advisable.
Dr. Seunghan Kim: What should be considered in a systemic approach for HER2-positive breast cancer with brain metastasis?
Dr. Jieun Lee: In cases of HER2-positive breast cancer with brain metastases, consideration of the CNS activity of systemic treatment is necessary. Anti-HER2 agents can be categorized into small molecular tyrosine kinase inhibitors (TKIs) and monoclonal antibodies. Among these, TKIs, due to their lower molecular weight, are characterized by the potential to penetrate the blood-brain barrier (BBB). Several studies have reported that, in HER2-positive breast cancer with stable brain metastasis, TKIs like lapatinib or neratinib, when combined with the cytotoxic agent capecitabine, showed an intracranial response rate of 20–66%.
78 However, evidence of the effectiveness of these agents in active brain metastasis has been limited. However, the phase II HER2CLIMB trial evaluating the efficacy of newer TKI, tucatinib included 291 patients with stable or active brain metastases at baseline. Among these, in 75 assessable patients, the combination of tucatinib with capecitabine and trastuzumab showed an intracranial response rate of 47.3%, indicating it could be a treatment option for active brain metastases.
9
On the other hand, monoclonal antibodies such as trastuzumab exhibit minimal penetration into the brain through the BBB under normal conditions due to their large molecular weight. However, when the BBB is disrupted during metastasis and becomes the blood-tumor barrier, it becomes more permeable to large molecules.
10
Some studies have shown that metastatic brain lesions in HER2-positive breast cancer demonstrate uptake with 64Cu-DOTA-trastuzumab or 89Zr-trastuzumab.
1112 It is also known that the intracranial response of the T-DM1 is about 20%, suggesting that in cases with brain metastases, large molecules like trastuzumab or trastuzumab-based antibody-drug conjugate (ADC) appear to be able to cross the BBB.
13
Dr. Seunghan Kim: What is the mechanism of action of T-DXd, and what is the reason for its high intracranial response?
Dr. Kabsoo Shin: T-DXd is an ADC composed of a humanized anti-HER2 monoclonal antibody (trastuzumab) coupled to potent topoisomerase I inhibitor, deruxtecan by a tetrapeptide-based cleavable linker. It has a high drug-to-antibody ratio of 8.
14 When T-DXd binds to the HER2 receptors of cancer cells, it is internalized into the tumor cells and subsequently undergoes intracellular trafficking to the lysosome. In the lysosomes, proteases cleave the linkers, releasing deruxtecan, which then inhibits topoisomerase within the nucleus. Due to its lipophilic nature, deruxtecan can also diffuse to adjacent cancer cells, showing cytotoxic effect, a phenomenon known as the ‘bystander effect.’
14
Despite its large molecular weight, T-DXd is thought to be able to penetrate brain lesions due to changes in intracranial permeability caused by brain metastases. Additionally, the fact that deruxtecan itself is a potent, lipophilic payload capable of crossing the BBB might also be a reason for its high intracranial response.
In retrospective analysis of the HER2-positive breast cancer patients with brain metastases in DESTINY-Breast01, -02, and -03 trial, intracranial response rate of T-DXd was about 45% in both stable and active brain metastases.
15 The TUXEDO-1 trial explored the intracranial efficacy of T-DXd in HER2-positive breast cancer patients with active brain metastases. The trial showed an intracranial response rate of 73.3% in 15 patients.
16 In the DEBBRAH trial, nine patients with progressing brain metastases after local treatment were enrolled in one of the five cohorts.
17 In this trial, the intracranial response rate of T-DXd was 44.4%.
Dr. Kabsoo Shin: The intracranial response of T-DXd shown in the previous studies is significantly effective compared to other antibody-based treatments, and the effect on active brain metastases should be particularly noted. However, in these trials, patients requiring immediate local intervention, and those with poor performance status were excluded. Although this case is an isolated example, it demonstrates that T-DXd can still be effective in active brain metastases, even with severe neurological deterioration and poor performance status. Additional evidence is necessary, but for patients with HER2-positive breast cancer who have brain metastases and are either unsuitable for WBRT or need to avoid WBRT’s neurocognitive decline side effects, considering treatments like T-DXd, as shown in this case, appears to be a viable option.
ACKNOWLEDGMENTS
The case conference section is prepared from monthly case conference of Department of Internal Medicine, the Catholic University of Korea College of Medicine, Seoul, Korea.
References
1. Le Rhun E, Guckenberger M, Smits M, Dummer R, Bachelot T, Sahm F, et al. EANO-ESMO clinical practice guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol. 2021; 32(11):1332–1347. PMID:
34364998.
2. Ramakrishna N, Anders CK, Lin NU, Morikawa A, Temin S, Chandarlapaty S, et al. Management of advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO guideline update. J Clin Oncol. 2022; 40(23):2636–2655. PMID:
35640075.
3. Schiff D, Messersmith H, Brastianos PK, Brown PD, Burri S, Dunn IF, et al. Radiation therapy for brain metastases: ASCO guideline endorsement of ASTRO guideline. J Clin Oncol. 2022; 40(20):2271–2276. PMID:
35561283.
4. Martin AM, Cagney DN, Catalano PJ, Warren LE, Bellon JR, Punglia RS, et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol. 2017; 3(8):1069–1077. PMID:
28301662.
5. Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008; 113(10):2638–2645. PMID:
18833576.
6. Pieńkowski T, Zielinski CC. Trastuzumab treatment in patients with breast cancer and metastatic CNS disease. Ann Oncol. 2010; 21(5):917–924. PMID:
19717536.
7. Bachelot T, Romieu G, Campone M, Diéras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013; 14(1):64–71. PMID:
23122784.
8. Freedman RA, Gelman RS, Anders CK, Melisko ME, Parsons HA, Cropp AM, et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2019; 37(13):1081–1089. PMID:
30860945.
9. Lin NU, Murthy RK, Abramson V, Anders C, Bachelot T, Bedard PL, et al. Tucatinib vs placebo, both in combination with trastuzumab and capecitabine, for previously treated ERBB2 (HER2)-positive metastatic breast cancer in patients with brain metastases: updated exploratory analysis of the HER2CLIMB randomized clinical trial. JAMA Oncol. 2023; 9(2):197–205. PMID:
36454580.
10. Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020; 20(1):26–41. PMID:
31601988.
11. Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010; 87(5):586–592. PMID:
20357763.
12. Kurihara H, Hamada A, Yoshida M, Shimma S, Hashimoto J, Yonemori K, et al. (64)Cu-DOTA-trastuzumab PET imaging and HER2 specificity of brain metastases in HER2-positive breast cancer patients. EJNMMI Res. 2015; 5(1):8. PMID:
25853014.
13. Montemurro F, Delaloge S, Barrios CH, Wuerstlein R, Anton A, Brain E, et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Ann Oncol. 2020; 31(10):1350–1358. PMID:
32634611.
14. Swain SM, Shastry M, Hamilton E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov. 2023; 22(2):101–126. PMID:
36344672.
15. Hurvitz S, Modi S, Li W, Park Y, Chung W, Kim S, et al. 377O A pooled analysis of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-positive (HER2+) metastatic breast cancer (mBC) with brain metastases (BMs) from DESTINY-Breast (DB)-01,-02, and-03. Ann Oncol. 2023; 34:S335–S336.
16. Bartsch R, Berghoff AS, Furtner J, Marhold M, Bergen ES, Roider-Schur S, et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med. 2022; 28(9):1840–1847. PMID:
35941372.
17. Pérez-García JM, Vaz Batista M, Cortez P, Ruiz-Borrego M, Cejalvo JM, de la Haba-Rodriguez J, et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: the DEBBRAH trial. Neuro Oncol. 2023; 25(1):157–166. PMID:
35639825.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download