1. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017; 151(1):193–203. PMID:
27780786.
2. Wang C, Wu Y, Shao J, Liu D, Li W. Clinicopathological variables influencing overall survival, recurrence and post-recurrence survival in resected stage I non-small-cell lung cancer. BMC Cancer. 2020; 20(1):150. PMID:
32093621.
3. Yun JK, Lee HP, Lee GD, Kim HR, Kim YH, Kim DK, et al. Recent trends in demographics, surgery, and prognosis of patients with surgically resected lung cancer in a single institution from Korea. J Korean Med Sci. 2019; 34(45):e291. PMID:
31760712.
4. Ekeke CN, Mitchell C, Schuchert M, Dhupar R, Luketich JD, Okusanya OT. Early distant recurrence in patients with resected stage I lung cancer: a case series of “Blast Metastasis”. Clin Lung Cancer. 2021; 22(1):e132–e135. PMID:
33144072.
5. Masago K, Seto K, Fujita S, Sasaki E, Hosoda W, Kuroda H. Long-term recurrence of completely resected NSCLC. JTO Clin Res Rep. 2020; 1(3):100076. PMID:
34589953.
6. Seung SJ, Hurry M, Walton RN, Evans WK. Real-world treatment patterns and survival in stage IV non-small-cell lung cancer in Canada. Curr Oncol. 2020; 27(4):e361–e367. PMID:
32905294.
7. Miller M, Hanna N. Advances in systemic therapy for non-small cell lung cancer. BMJ. 2021; 375(2363):n2363. PMID:
34753715.
8. Park S. Dancing with the surgeon: neoadjuvant and adjuvant immunotherapies from the medical oncologist’s perspective. J Chest Surg. 2023; 56(2):67–74. PMID:
36864673.
9. Jung JM, Hyun SJ, Kim KJ. Surgical impacts of metastatic non-small cell lung cancer to the thoracic and lumbar spine. J Korean Med Sci. 2021; 36(7):e52. PMID:
33619918.
10. Reck M, Remon J, Hellmann MD. First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol. 2022; 40(6):586–597. PMID:
34985920.
11. Brahmer JR, Lee JS, Ciuleanu TE, Bernabe Caro R, Nishio M, Urban L, et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in CheckMate 227. J Clin Oncol. 2023; 41(6):1200–1212. PMID:
36223558.
12. Isbell JM, Li BT, Gomez DR. The emerging role of local therapy in oligometastatic non-small cell lung cancer. J Thorac Cardiovasc Surg. 2022; 163(3):819–825. PMID:
34147255.
13. Yoo S, Cho WC, Lee GD, Choi S, Kim HR, Kim YH, et al. Long-term surgical outcomes in oligometastatic non-small cell lung cancer: a single-center study. J Chest Surg. 2023; 56(1):25–32. PMID:
36517949.
14. Jung YJ, Oh IJ, Kim Y, Jung JH, Seok M, Lee W, et al. Clinical validation of a protein biomarker panel for non-small cell lung cancer. J Korean Med Sci. 2018; 33(53):e342. PMID:
30595683.
15. Kim DJ, Kim WJ, Lim M, Hong Y, Lee SJ, Hong SH, et al. Plasma CRABP2 as a novel biomarker in patients with non-small cell lung cancer. J Korean Med Sci. 2018; 33(26):e178. PMID:
29930489.
16. Lee S, Kang HG, Choi JE, Lee JH, Kang HJ, Baek SA, et al. The different effect of VEGF polymorphisms on the prognosis of non-small cell lung cancer according to tumor histology. J Korean Med Sci. 2016; 31(11):1735–1741. PMID:
27709850.
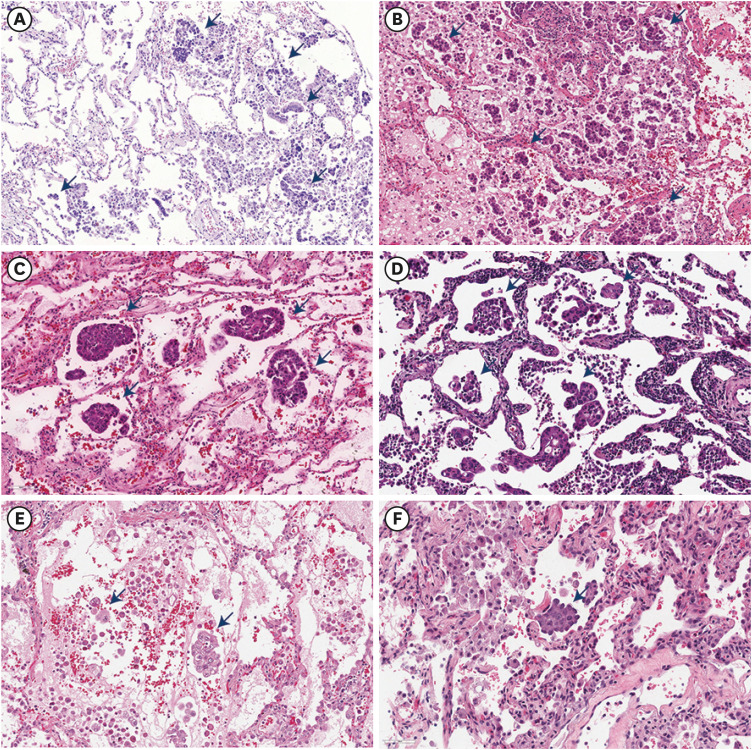
17. Warth A, Muley T, Kossakowski CA, Goeppert B, Schirmacher P, Dienemann H, et al. Prognostic impact of intra-alveolar tumor spread in pulmonary adenocarcinoma. Am J Surg Pathol. 2015; 39(6):793–801. PMID:
25723114.
18. Kadota K, Nitadori JI, Sima CS, Ujiie H, Rizk NP, Jones DR, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol. 2015; 10(5):806–814. PMID:
25629637.
19. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press;2015.
20. Yanagawa N, Shiono S, Endo M, Ogata SY. Tumor spread through air spaces is a useful predictor of recurrence and prognosis in stage I lung squamous cell carcinoma, but not in stage II and III. Lung Cancer. 2018; 120:14–21. PMID:
29748009.
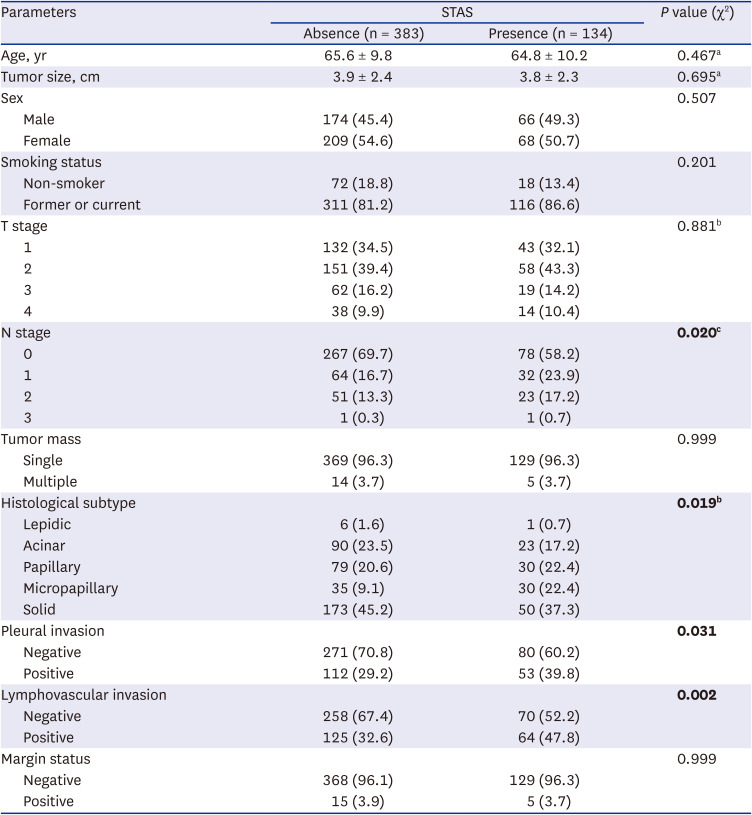
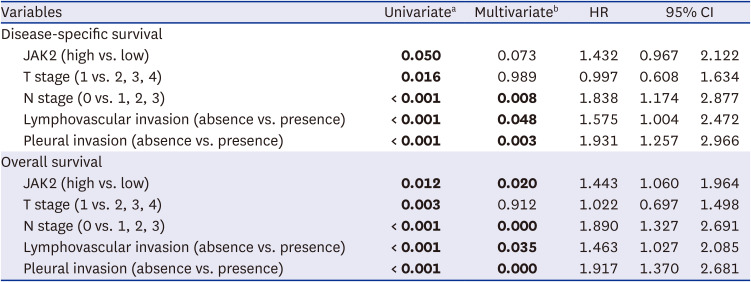
21. Han YB, Kim H, Mino-Kenudson M, Cho S, Kwon HJ, Lee KR, et al. Tumor spread through air spaces (STAS): prognostic significance of grading in non-small cell lung cancer. Mod Pathol. 2021; 34(3):549–561. PMID:
33199839.
22. Sim J, Kim H, Hyeon J, Choi Y, Han J. Anaplastic lymphoma kinase (ALK)-expressing lung adenocarcinoma with combined neuroendocrine component or neuroendocrine transformation: implications for neuroendocrine transformation and response to ALK-tyrosine kinase inhibitors. J Korean Med Sci. 2018; 33(15):e123. PMID:
29629521.
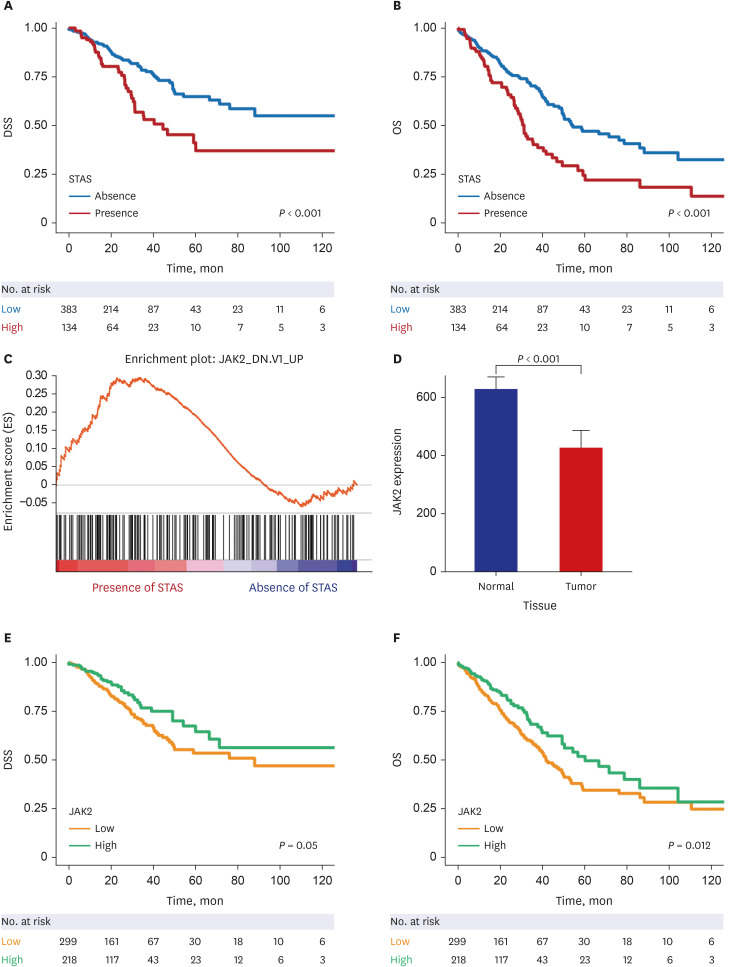
23. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005; 102(43):15545–15550. PMID:
16199517.
24. Bindea G, Galon J, Mlecnik B. CluePedia Cytoscape plugin: pathway insights using integrated experimental and in silico data. Bioinformatics. 2013; 29(5):661–663. PMID:
23325622.
25. Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009; 25(8):1091–1093. PMID:
19237447.
26. Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018; 24(10):1550–1558. PMID:
30127393.
27. Yoshizawa A, Motoi N, Riely GJ, Sima CS, Gerald WL, Kris MG, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011; 24(5):653–664. PMID:
21252858.
28. Jung WY, Min KW, Oh YH. Increased VEGF-A in solid type of lung adenocarcinoma reduces the patients’ survival. Sci Rep. 2021; 11(1):1321. PMID:
33446784.
29. Yang W, Soares J, Greninger P, Edelman EJ, Lightfoot H, Forbes S, et al. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013; 41:D955–D961. PMID:
23180760.
30. Blaauwgeers H, Flieder D, Warth A, Harms A, Monkhorst K, Witte B, et al. A prospective study of loose tissue fragments in non–small cell lung cancer resection specimens: an alternative view to “spread through air spaces”. Am J Surg Pathol. 2017; 41(9):1226–1230. PMID:
28622180.
31. Thunnissen E, Blaauwgeers HJ, de Cuba EM, Yick CY, Flieder DB. Ex vivo artifacts and histopathologic pitfalls in the lung. Arch Pathol Lab Med. 2016; 140(3):212–220. PMID:
26927715.
32. Ikezoe T, Kojima S, Furihata M, Yang J, Nishioka C, Takeuchi A, et al. Expression of p-JAK2 predicts clinical outcome and is a potential molecular target of acute myelogenous leukemia. Int J Cancer. 2011; 129(10):2512–2521. PMID:
21207414.
33. Sonohara F, Nomoto S, Inokawa Y, Hishida M, Takano N, Kanda M, et al. High expression of Janus kinase 2 in background normal liver tissue of resected hepatocellular carcinoma is associated with worse prognosis. Oncol Rep. 2015; 33(2):767–773. PMID:
25420511.
34. Song Y, Tang MY, Chen W, Wang Z, Wang SL. High JAK2 protein expression predicts a poor prognosis in patients with resectable pancreatic ductal adenocarcinoma. Dis Markers. 2020; 2020:7656031. PMID:
33029256.
35. Economopoulou P, Kotoula V, Koliou GA, Papadopoulou K, Christodoulou C, Pentheroudakis G, et al. Prognostic impact of Src, CDKN1B, and JAK2 expression in metastatic breast cancer patients treated with trastuzumab. Transl Oncol. 2019; 12(5):739–748. PMID:
30877976.
36. Ikeda S, Okamoto T, Okano S, Umemoto Y, Tagawa T, Morodomi Y, et al. PD-L1 is upregulated by simultaneous amplification of the PD-L1 and JAK2 genes in non-small cell lung cancer. J Thorac Oncol. 2016; 11(1):62–71. PMID:
26762740.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download