This article has been
cited by other articles in ScienceCentral.
Abstract
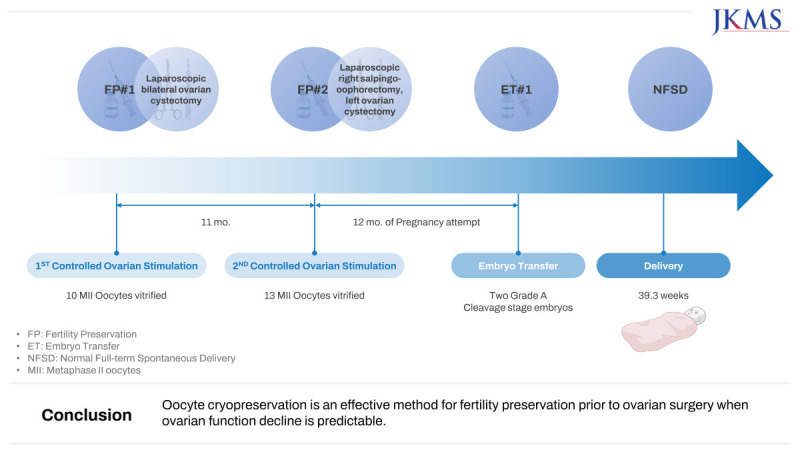
This article reports the live birth of a healthy newborn using vitrified-warmed oocytes from fertility preservation before ovarian surgery. The patient in our case underwent two cycles of controlled ovarian stimulation before laparoscopic bilateral ovarian cystectomy for endometriosis, and a total of 23 mature oocytes were vitrified. After surgery, her pathologic reports revealed a serous borderline tumor and endometrioma. Fifteen months after her second surgery of laparoscopic right salpingo-oophorectomy and left ovarian cystectomy owing to recurrence, she had been married by then, and three of the frozen oocytes were thawed for intracytoplasmic sperm injection. These oocytes were cryopreserved for 2.5 years. All three were fertilized, and two grade-A cleavage-stage embryos were transferred. A singleton pregnancy was achieved, resulting in the delivery of a healthy baby boy at 39.3 weeks of gestation. Oocyte cryopreservation is an effective method for fertility preservation prior to ovarian surgery when ovarian function decline is predictable.
Keywords: Borderline Ovarian Tumor, Fertility Preservation, Intracytoplasmic Sperm Injection, Oocyte Cryopreservation, Pregnancy
INTRODUCTION
Fertility preservation before bilateral ovarian surgery has become of increasing interest within the past decade, especially due to the accumulation of reports showing successful pregnancy using vitrified-warmed oocytes.
123 Vitrification of mature oocytes has emerged as an established method for fertility preservation in women requiring treatment that pose a serious threat to their future fertility, which has also been supported by the American Society of Reproductive Medicine (ASRM).
4 In vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) using cryopreserved oocytes have shown fertilization and pregnancy rates comparable to using fresh oocytes.
5
Even with such encouraging results regarding IVF with cryopreserved oocytes, unfortunately, its proposal to patients before gonadotoxic treatments is still insufficient. The referral rate of oncology patients to specialists for fertility preservation prior to cancer therapy is still low.
6 Moreover, fertility preservation is equally essential in patients with a high risk of fertility decline, including patients with endometriosis or those undergoing ovarian surgery.
7 The reduction in the ovarian reserve after surgery is unpredictable in each patient.
Here, we report the successful outcome of a pregnancy and delivery following the use of a vitrified-warmed oocyte for IVF and embryo transfer (ET) in a woman who was offered oocyte cryopreservation for fertility preservation before bilateral ovarian surgery due to a borderline ovarian tumor (BOT).
CASE DESCRIPTION
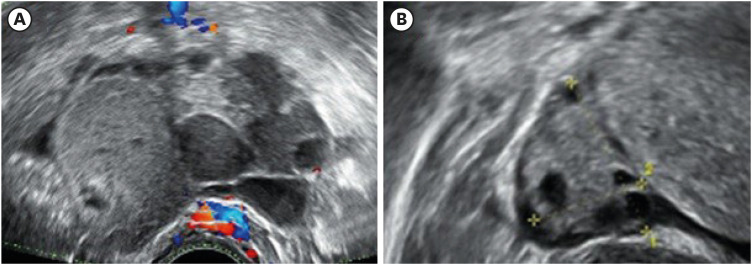
In July 2018, a 26-year-old single woman was referred to our center for the further evaluation of a quadrilocular right ovarian cyst measuring up to 7 cm on transvaginal ultrasonography (TV-USG) (
Fig. 1). At the time, endometrioma of the right ovary was suspected. This finding was accompanied by an elevated serum marker cancer antigen-125 level of 88.0 U/mL. Her anti-müllerian hormone (AMH) level was 2.62 ng/mL and antral follicle count (AFC) of 11. We planned to perform laparoscopic ovarian cystectomy, and the possibility of post-surgical ovarian function decline was informed to the patient. Before her treatment, oocyte cryopreservation was offered, which she strongly desired.
Fig. 1
The patient’s transvaginal-ultrasonography image of (A) the quadrilocular right ovarian cyst measuring up to 7 cm and (B) the normal-looking left ovary.
In August 2018, the patient underwent a cycle of controlled ovarian stimulation (COS) with gonadotropin-releasing hormone antagonist protocol. When the leading follicle of the left ovary measured 18 mm, final oocyte maturation was triggered by recombinant human chorionic gonadotrophin (Ovidrel 250 mcg, Merck-Serono). TV-USG-guided oocyte retrieval was carried out 36 hours later, yielding 10 mature metaphase-II (MII) oocytes which were then vitrified using the Kitazato Vitrification media and Cryotop (Kitazato, Fuji, Japan) according to the manufacturer’s protocol. There were no procedure-related complications.
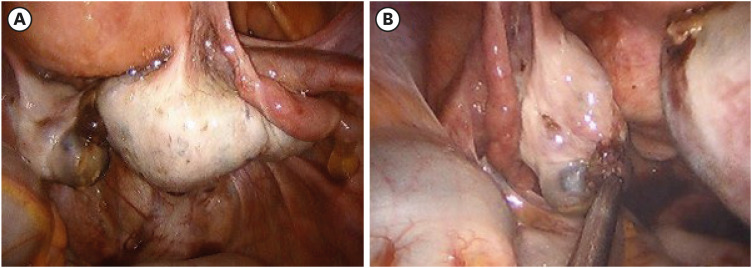
Eighteen days later, the patient underwent surgery as planned. Upon entering the abdominal cavity, bilateral ovarian cysts were noted, and laparoscopic bilateral ovarian cystectomy was performed (
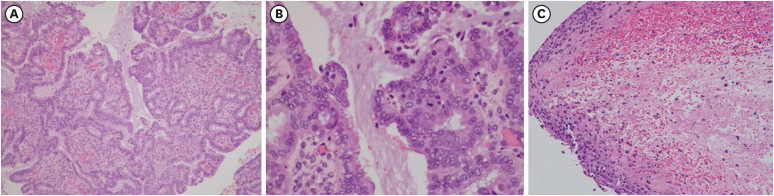
Fig. 2). Final pathologic results showed a serous borderline tumor on the right side and an endometrioma on the left side (
Fig. 3).
Fig. 2
Intra-operative findings of the first operation (laparoscopic bilateral ovarian cystectomy) (A) of the right ovary and (B) of the left ovary with incidental cystic finding.
Over the next year, she paid regular visits to our center and was checked for recurrence, and her 6 months post-operative AMH level measured 1.92 ng/mL. Meanwhile, she got married in February 2019. In July 2019, her magnetic resonance imaging scans showed suspicion of bilateral ovarian tumor recurrence, and therefore, a second surgery was planned. At that time, her AMH level was 1.61 ng/mL, and her AFC was 9. Before her second surgery, she desired to further preserve her oocytes. In November 2019, she underwent a second cycle of COS using the same medications as the first. This cycle yielded 13 MII oocytes which were also vitrified through the same method as the first; hence she had a total of 23 MII oocytes cryopreserved.
Two months later, the patient had the second surgery of laparoscopic right salpingo-oophorectomy and left ovarian cystectomy (
Fig. 4). The final pathology reported a recurrence of serous borderline tumor on the right side and a benign cyst without a lining epithelium but with old hemorrhage inside. Her AMH level three months after surgery was 2.02 ng/mL, and for the next year, she and her husband tried to conceive naturally and did not succeed for 12 months. In March 2021, the patient, now aged 29, requested the warming of oocytes for IVF. Out of the 23 cryopreserved MII oocytes, three were warmed from those cryopreserved in the first cycle (in 2018), which were cryopreserved for 2.5 years. Warming was performed according to the manufacturer’s instructions of the Kitazato Thawing media (Kitazato), and all of them survived. These were fertilized by ICSI with her husband’s sperm, and all 3 were fertilized. After three days, two eight-cell embryos of grade A and one of grade B were developed, but we decided only to transfer the two grade-A embryos. Transabdominal ultrasound-guided ET was performed. A single viable intrauterine pregnancy was confirmed by a fetal heartbeat on TV-USG. In November 2021, at 39.3 weeks of gestation, she gave birth to a healthy boy weighing 3.31 kg via normal full-term vaginal delivery.
Fig. 3
Pathology sections of (A) right ovarian cyst, which is consistent with serous borderline tumor (100×) (B) right ovarian cyst, serous borderline tumor at higher magnification (400×) (C) left ovarian cyst, consistent with endometrioma (100×).
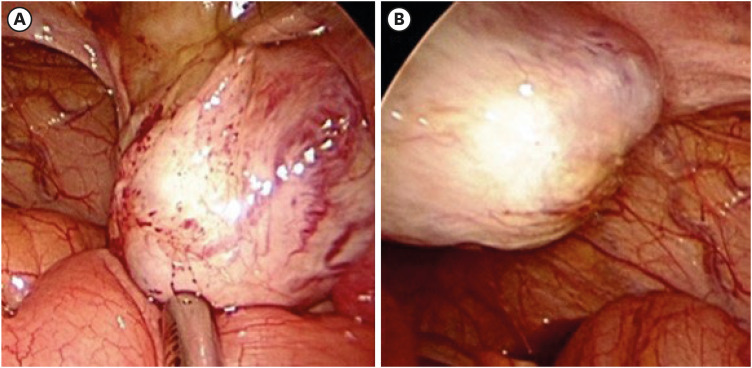
Fig. 4
Intra-operative findings of the second operation (laparoscopic right salpingo-oophorectomy and left ovarian cystectomy) (A) of the right ovary and (B) of the left ovary.
DISCUSSION
We present a successful case of pregnancy and delivery following ICSI of cryopreserved oocytes of a single young woman prior to bilateral ovarian surgery where ovarian function decline is predictable.
Recent improvements in vitrification techniques for oocyte cryopreservation have led to promising results concerning the efficacy and safety of vitrified-warmed oocytes. The study by Noyes et al.
8 showed that the delivery rate of IVF using vitrified oocytes is comparable to that of fresh oocytes. They also showed, in another study, promising perinatal outcomes of IVF using vitrified oocytes.
9 Such findings support vitrifying oocytes in women whose future fertility is threatened. Recently, ASRM announced that oocyte cryopreservation should no longer be considered experimental.
4
There are numerous studies that have shown successful live births using vitrified-warmed oocytes, but mainly in patients before gonadotoxic treatments.
123 One recent report showed a live birth from a frozen-thawed oocyte from a woman who had already undergone fertility-sparing surgery for BOT.
10 Kim et al.
7 reported that women with endometriomas should be counseled about oocyte cryopreservation for fertility preservation before surgery and that the number of cryopreserved oocytes can be increased by repeated oocyte retrieval. Cobo et al.
11 showed, through results obtained by 485 endometriosis patients, high pregnancy and live birth rates after vitrification. Our study is a case report to show a successful pregnancy from frozen-thawed oocytes collected for fertility preservation before the first and second surgery for recurrence.
Safety may be an issue in ovarian tumors, especially if it is a borderline or malignant case. Physicians should be extremely cautious during the oocyte retrieval process since there is always a possibility of tumor spillage. We did not puncture follicles adjacent to the tumor or in a difficult location, as our priority was not to rupture the cyst. Cytologic test with follicular fluid is currently not the standard practice, and no studies have investigated the cytology with follicular fluid and disease prognosis.
There is a lack of evidence investigating the effect of tumor spillage during oocyte retrieval and prognosis due to consequent up-staging. Recurrences are not uncommon, but how much could be contributed to COS and oocyte retrieval in relapse episodes is unclear.
12 Ovarian cyst did not increase after COS in our case, and it is quite unlikely existing cysts will increase further due to COS for several days.
Postoperative serum AMH level decreases after undergoing ovarian cystectomy for endometriosis, and this decreases by a greater extent in patients with bilateral endometrioma than with unilateral endometrioma.
13 Also, reduction in ovarian reserve after surgery is unpredictable in each patient, and fertility preservation should be carried out even when AMH level is relatively high.
7 As suggested by Cobo et al.,
14 the preservation of 12 vitrified oocytes prior to 35 years of age results in cumulative live birth rate of 61.9%.
One peculiar point with this patient is that the AMH after the second operation did not decline. It could be due to laboratory measurement variations. Moreover, follicular functions may have been activated temporarily after the surgery, leading to increased AMH value. It should be kept in mind that AMH is not the absolute indicator of the number of follicles remaining, and an increment in AMH value should not be interpreted as an actual increase of ovarian reserve after the surgery.
The overall risk of recurrence after conservative ovarian surgery, that is, unilateral salpingo-oophorectomy or ovarian cystectomy, is higher than bilateral salpingo-oophorectomy (0–25% vs. 0–5%, respectively).
15 Salpingo-oophorectomy is often required after ovarian cystectomy due to recurrence. Therefore, fertility preservation is highly essential even when fertility-sparing conservative surgery for BOT is undertaken. To reduce delayed treatment of the tumor, random-start ovarian stimulation can be considered to decrease the time of the COS.
16
We believe that it is still important to report such results in order to emphasize the importance of referring such single women to a reproductive endocrinologist when planning to perform bilateral ovarian surgery. We hope this report can provide valuable information for medical professionals encountering women who can benefit from oocyte preservation, especially before performing bilateral ovarian surgery for adnexal tumors.






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download