Abstract
The substantia gelatinosa (SG) within the trigeminal subnucleus caudalis (Vc) is recognized as a pivotal site of integrating and modulating afferent fibers carrying orofacial nociceptive information. Although naringenin (4',5,7-thrihydroxyflavanone), a natural bioflavonoid, has been proven to possess various biological effects in the central nervous system (CNS), the activity of naringenin at the orofacial nociceptive site has not been reported yet. In this study, we explored the influence of naringenin on GABA response in SG neurons of Vc using whole-cell patch-clamp technique. The application of GABA in a bath induced two forms of GABA responses: slow and fast. Naringenin enhanced both amplitude and area under curve (AUC) of GABA-mediated responses in 57% (12/21) of tested neurons while decreasing both parameters in 33% (7/21) of neurons. The enhancing or suppressing effect of naringenin on GABA response have been observed, with enhancement occurring when the GABA response was slow, and suppression when it was fast. Furthermore, both the enhancement of slower GABA responses and the suppression of faster GABA responses by naringenin were concentration dependent. Interestingly, the nature of GABA response was also found to be sex-dependent. A majority of SG neurons from juvenile female mice exhibited slower GABA responses, whereas those from juvenile males predominantly displayed faster GABA responses. Taken together, this study indicates that naringenin plays a partial role in modulating orofacial nociception and may hold promise as a therapeutic target for treating orofacial pain, with effects that vary according to sex.
The trigeminal subnucleus caudalis (Vc), also called medullary dorsal horn due to its homologous structure with the spinal dorsal horn, is known to be a major relay site for orofacial nociceptive inputs [1,2]. The sensory nucleus of the spinal cord, substantia gelatinosa (SG; lamina II), was named by Rolando for its translucent, gelatinous appearance [3]. SG neurons are commonly reported to receive incoming painful information and transmit it to ascending neurons [4]. In particular, SG neurons in Vc are known to play an important role in transmitting orofacial nociception to higher brain centers via thin myelinated Aδ and unmyelinated C primary afferent fibers [5].
Orofacial pain is frequently and severely triggered by the abundance of sensory receptors located in skin, mucous membranes, muscles, and ligaments of face [6,7]. For example, orofacial diseases such as trigeminal neuralgia, temporomandibular joint disorder, atypical toothache, and headache cause severe acute and chronic pain despite various treatments and reduce quality of life [7]. GABA is a major inhibitory neurotransmitter in the central nervous system (CNS) and is known to act as a nociceptive modulator in the spinal cord and human brain [8]. Additionally, many reported studies have shown that GABA can enhance or reduce pain transmission and perception due to differences in the composition of GABA receptor subunits [9,10].
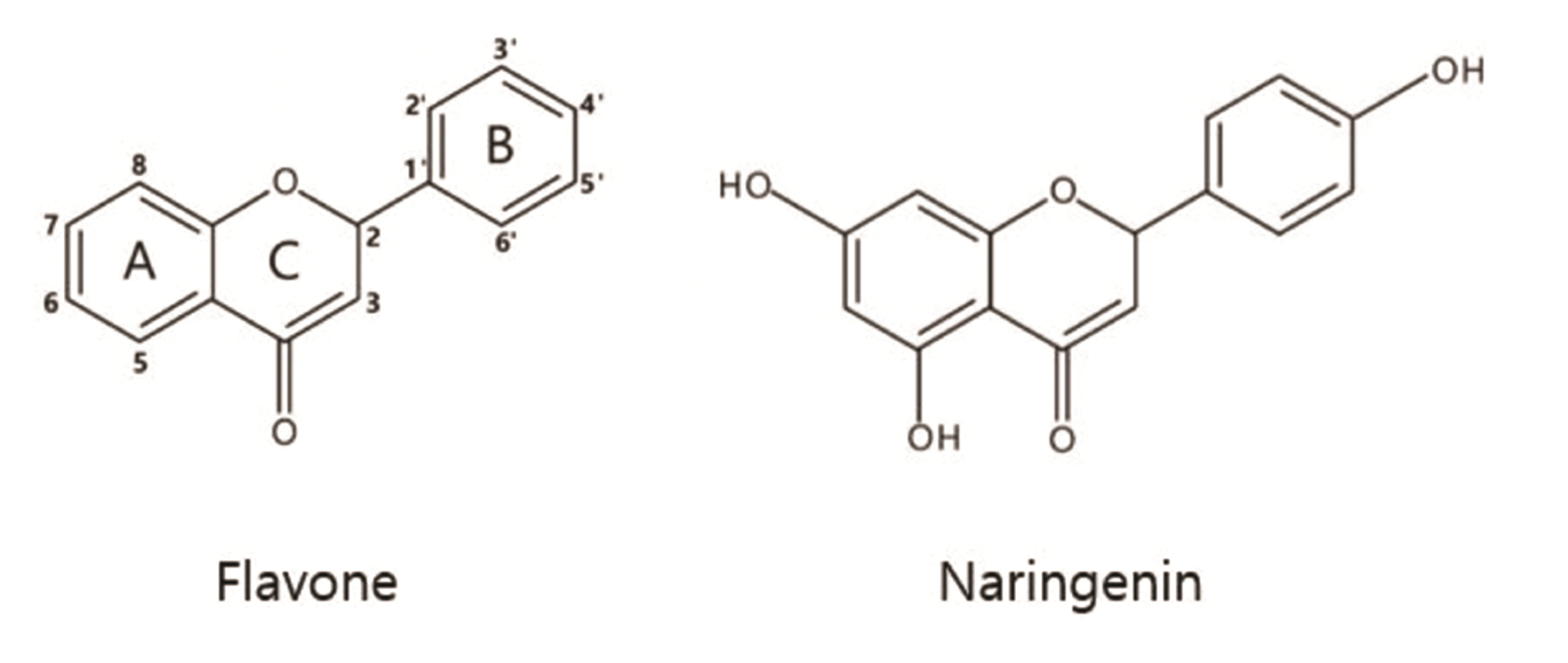
The orofacial anti-nociceptive mechanisms of natural compounds related to neuromodulation have been studied over the past few decades [11], among which the prominent flavanone, (4',5,7-thrihydroxyflavanone (naringenin; Fig. 1) is majorly found in grapefruit and some other fruits and herbs, such as sour orange, tart cherries, tomatoes, cocoa, Greek oregano, and even beans [12,13]. Owing to its good bioavailability, naringenin is easily detected in human serum after intaking [14]. Interestingly, naringenin possesses anti-oxidative competence via protecting lipids against oxidative damage [15]. Furthermore, a previous study has shown that naringenin can advance memory function and reduce amyloid plaques and tau proteins in an Alzheimer’s disease model [16]. Naringenin also shows analgesic and anti-inflammatory activities through inhibiting pain-like behavior produced by inflammatory stimuli [17]. Although naringenin has been demonstrated to modulate nociceptive pain [18,19], role of naringenin in orofacial pain perception has not been reported yet. Therefore, the purpose of this study was to investigate the effect of naringenin and related mechanism on SG neurons of the Vc using the whole-cell patch-clamp technique and thereby gaining insight into its role in orofacial pain perception.
Experiments were approved by the Institutional Animal Care and Use Committee of Jeonbuk National University (CBNU 2020-0122) and performed in accordance with the guidelines for the Care and Use of Laboratory Animals issued by Jeonbuk National University. Immature ICR mice postnatal days (PND) 7 to 21 were housed under 12-h light/dark cycles. They were allowed free access to food and water ad libitium (lights on at 7:00). Because the inhibitory effect in dorsolateral funiculus stimulation descends when spinal dorsal horn neurons closely approach to adult condition (from three postnatal weeks) [20,21], we referred the point of three weeks aging as a dividing point of maturity in SG neurons of mice.
Brain slices were prepared as described in our previous studies [22]. Mice were decapitated between 10:00 AM to 12:00 PM UTC + 9:00 (Universal Time Coordinate). Their brains were immediately excised and rapidly immersed in ice-cold artificial cerebrospinal fluid (ACSF) with the following compounds (in mM): 126 NaCl, 2.5 KCl, 2.4 CaCl2, 1.2 MgCl2, 11 D-glucose, 1.4 NaH2PO4, and 25 NaHCO3 (pH 7.3–7.4, bubbled with 95% O2 and 5% CO2). To prepare coronal slices (180–200 µm in thickness), brainstem containing the rostral part of Vc was glued to an agar block and then, cut in ice-cold ACSF with a vibratome (Leica VT1200S; Leica Biosystems). Before electrophysiological recordings, these slices were allowed to recover in oxygenated ACSF for a minimum of one hour. All experimental steps were performed at room temperature.
Brain slices were transferred to a recording chamber, which was continuously superfused with oxygenated ACSF at 4–5 ml/min. Slices were viewed on the stage of an upright microscope (BX51W1; Olympus) with differential interference contrast optics. Patch pipettes were pulled from borosilicate glass capillaries (PG52151-4; WPI) using a Flaming/Brown micropipette puller (P-97; Sutter Instruments Co.). A high chloride-based internal solution, consisting of 140 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 4 mM Mg-ATP, and 10 EGTA (pH 7.3 with KOH) was filtered through a disposable 0.22 µm filter and loaded into the pipette.
Under the microscope’s magnification, the SG (lamina II) was apparently distinguished as a translucent band, just medial to the spinal trigeminal tract crossing the lateral edge of the brain slice. Tip resistance of recording pipettes ranged from 4 to 6 MΩ when loaded with a high chloride-based internal solution. Once a gigaseal was achieved between SG neuron and the pipette, neurons were voltage-clamped at –60 mV and current-clamped for whole-cell patch-clamp recording. An intact neuronal membrane was ruptured by a short suction for whole-cell patch-clamp recordings [23].
Signals were amplified and filtered at 1 kHz with an Axopatch 200B (Molecular Devices) and digitized using a Digidata 1440A (Molecular Devices). Acquisition and subsequent analysis of the recorded data were done using Clampex 10.6 software (Molecular Devices). The validity of this process was confirmed by once more visual analysis of all traces before being approved for further analysis in Origin software (OriginLab Corp.).
Naringenin, GABA and chemicals for ACSF were purchased from Sigma Aldrich. Stocked chemicals were prepared according to their solubility in dimethyl sulfoxide (DMSO) or distilled water and diluted to tested concentration in ACSF shortly before being bath applied.
To determine relative changes, the target response was divided by its corresponding control response. SG neurons were considered affected if the presence of naringenin resulted in a change of more than 10% compared to the control response. For area under the curve (AUC) measurement, the negative pA value was converted to a positive value, and then the mean value of 1-min period before GABA application was established as the baseline mean value. Traces recovered within 6 min from the time of GABA in presence of naringenin were utilized for AUC calculation. Each data point was normalized to the baseline mean value and Prism 10's AUC calculation method was employed to determine the AUC.
The means of two groups were compared using either paired or unpaired Student’s t-test, and for comparisons involving multiple groups, a one-way ANOVA followed by the post-hoc Scheffe test was applied. All numerical values are expressed as the mean ± standard error of the mean (SEM). Statistical significance was defined as p < 0.05 and the levels of significance were defined by asterisks (*p < 0.05, **p < 0.01, and ***p < 0.005).
To confirm the effect of naringenin, various concentrations (ranging from 1 µM to 2 mM) were bath applied to SG neurons in voltage clamp mode. Bath application of naringenin induced weak inward current with the concentration 1 mM and greater (Fig. 2A, B, n = 10). Subsequently, the effect of 0.5 mM naringenin on holding current was assessed, revealing no significant impact (Fig. 2C, D, n = 3). DMSO, used as a vehicle for naringenin, also demonstrated no effect on membrane current (figure not shown). Next, bath application of 30 µM GABA for 3 min in SG neurons induced inward currents of two distinct characteristics, i.e., slow response (Fig. 2E) and fast response (Fig. 2F). The average time for the slow GABA response was 112.2 ± 4.96 sec (n = 15), while it was 40.2 ± 4.08 sec (n = 6) for fast response. The mean amplitude of slow GABA response was –52.6 ± 9.84 pA (n = 15), and the fast GABA response had a mean amplitude of –53.7 ± 18.06 pA (n = 6). However, repeated application of both slow (Fig. 2G, n = 15) and fast (Fig. 2H, n = 6) GABA-induced response showed no significant differences (total, Fig. 2I; n = 21, p > 0.05). The results in Fig. 2E–I suggest that the response of increasing or decreasing in the subsequent process is attributable to the concurrent application of naringenin with GABA, as there is no significant change observed with repeated application.
To investigate the effect of naringenin on GABA response, GABA was first bath applied and washed out for 5 min. Next, naringenin 0.5 mM was bath applied for 3 min and then coapplied with GABA. As shown in Fig. 3, 30 µM GABA-induced slow response was increased in 57% of neurons tested (Fig. 3A, n = 12/21). In this case, the mean relative amplitude and AUC were significantly increased to 1.70 ± 0.185 (Fig. 3B; n = 12, p < 0.01) and 1.88 ± 0.265 (Fig. 3C; n = 12, p < 0.01), respectively in the presence of naringenin. Next, naringenin decreased the GABA-induced fast response in 33% of SG neurons tested (Fig. 3D, n = 7/21). In this case, the mean relative amplitude and AUC were significantly decreased to 0.589 ± 0.050 (Fig. 3E; n = 7, p < 0.001) and 0.757 ± 0.075 (Fig. 3F; n = 7, p < 0.05), respectively in the presence of naringenin. In two neurons, no significant change was observed even in the presence of naringenin (n = 2/21, 10%, data not shown).
As observed in Fig. 3, naringenin exhibited varied effects on GABA induced inward current depending upon the nature of GABA response. Fast GABA responses were suppressed while slow GABA responses were enhanced by 0.5 mM naringenin. Next concentration dependent effect of naringenin was assessed in both form of GABA response. Naringenin concentration ranging from 1 µM to 1 mM exhibited concentration dependent effect on both slow and fast GABA responses. Slow GABA response was enhanced (Fig. 4A) while fast GABA response was suppressed (Fig. 4B) with increasing concentration of naringenin. Fig. 4C and D represent the histograms showing the increasing and decreasing tendency of slow GABA response and fast GABA response respectively, with the increasing concentration of naringenin. The relative amplitude of GABA-induced inward currents exhibited distinct patterns in response to naringenin concentration. Specially, for slow GABA responses, the relative amplitudes were, naringenin 1 µM: 1.39 ± 1.06; 10 µM: 2.17 ± 0.950; 100 µM: 2.83 ± 1.55; 1 mM: 2.87 ± 1.09 (Fig. 4C, n = 4, p < 0.05; one-way ANOVA). In contrast, for fast GABA responses, the relative amplitudes demonstrated a decreasing trend with increasing naringenin concentration, naringenin 1 µM: 1.00 ± 0.088; 10 µM: 0.96 ± 0.244; 100 µM: 0.74 ± 0.221; 1 mM: 0.23 ± 0.086 (Fig. 4D, n = 5, p < 0.01; one-way ANOVA).
To confirm that naringenin could affect membrane potential, GABA was applied in the presence of naringenin in current-clamp mode (Fig. 5). Naringenin itself did not influence membrane potential but enhanced the durability of GABA response as shown in Fig. 5A. Initially, GABA was bath-applied for 2-min, followed by a 5-min washout period, and then 0.5 mM naringenin was applied for 2 min before co-application with GABA. The GABA-induced depolarization did not exhibit a significant change, with a mean relative amplitude of 0.92 ± 0.130 (Fig. 5B, n = 5, p > 0.05). However, the mean relative AUC significantly increased to 1.23 ± 0.039 in the presence of naringenin (Fig. 5C, n = 5, p < 0.05). This result suggests that naringenin may have an enhancing effect on the inhibitory GABA receptors known to modulate SG neurons of the Vc.
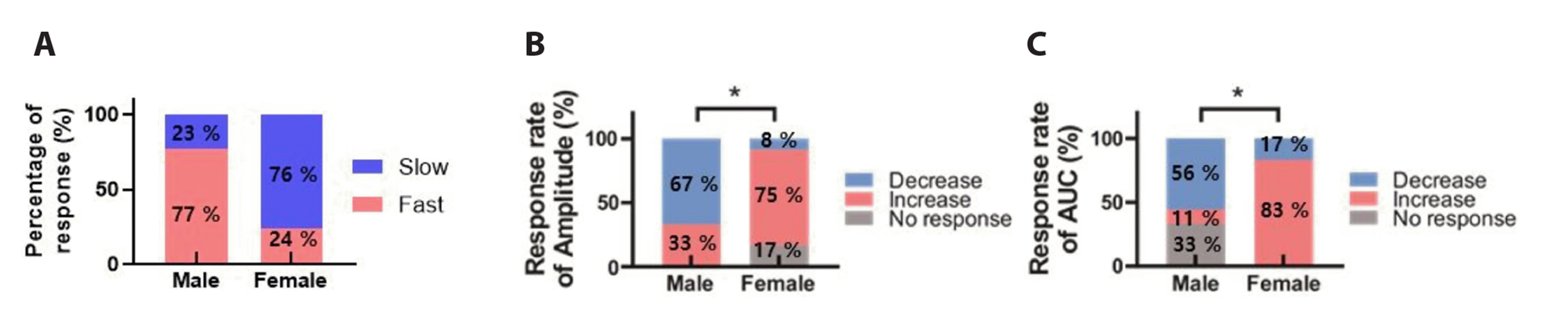
As shown in Fig. 6A, further analysis of the voltage-clamped recorded data revealed that most SG neurons from males (10/13, 77%) showed fast GABA response and remaining neurons (3/13, 23%) showed slow response. In contrast, most SG neurons (13/17, 76%) from female juvenile mice showed a slow GABA response upon bath application of 30 µM GABA, while the remaining neurons (4/17, 24%) displayed a fast GABA response.
As previously indicated in the results, naringenin inhibits fast GABA responses and enhances slow GABA responses. Among males, 67% of the tested SG neurons (6/9) exhibited a decrease in GABA response amplitude upon exposure to naringenin, while the remaining three neurons showed an increase. Conversely, in females, the application of 0.5 mM naringenin led to an increased amplitude of the GABA response in 75% of the tested SG neurons (9/12), with one neuron displaying a decrease, and no effect observed in the remaining two neurons (Fig. 6B). Additionally, naringenin predominantly reduced the AUC of GABA-induced inward currents in most of the tested neurons (56%, 5/9), with one neuron showing an increase, and the remaining three neurons remaining unaffected in males. In contrast, most SG neurons (83%, 10/12) from females demonstrated an increase in the AUC of the GABA-induced response in the presence of 0.5 mM naringenin, while the remaining two neurons displayed a decrease (Fig. 6C). This suggests that naringenin’s impact on SG neurons is influenced by the nature of the GABA response and exhibits a sex-dependent pattern.
In the present study, we observed that naringenin exerted an influence on GABA receptor-mediated responses within the SG neurons in the Vc. Notably, naringenin potentiated GABA receptor-mediated responses in the majority of SG neurons showing slower GABA responses, while it inhibited such responses in a minority of neurons exhibiting faster GABA response. Importantly, both the potentiation and inhibition of GABA receptor-mediated responses were found to be dependent upon the concentration of naringenin. Furthermore, our results underscore the sex-dependent nature of naringenin’s effect on GABA responses. These findings highlight the significant influences of the nature of the GABA response itself in determining the effects of naringenin on it.
This study focuses on SG neurons in the dorsal horn (lamina II) of the spinal cord, recognized as the central gate for modulating nociception information [24,25]. Laminae I-III of the dorsal horn contains numerous inhibitory interneurons that regulate sensory information before transmitting it to other parts of the brain and spinal cord. Most neurons in layers I-III are glycine-rich, and it is reported that these inhibitory interneurons also contain GABA. These interneurons constitute approximately 30% of laminae I-II neurons and about 40% of laminae III neurons. Immunocytochemical studies confirm their GABAergic nature, with some using glycine as a co-transmitter [24,26]. Several reports indicate that the development of the nervous system can vary based on sex, leading to diverse nerve structures [27]. Additionally, changes in GABA receptor responsiveness may occur depending on sex or age [28,29]. Chudomel et al. [28], observed higher GABAA receptor-mediated sIPSC amplitude and frequency in male rats compared to females in the substantia nigra reticulate, with rise and decay time decreasing with age. This variation according to sex and age may depend on the heterogeneity on the GABAA receptor subunit composition [22].
GABA receptors are pentameric protein complexes composed of five subunits assembled from the pool of 19 different subunits (α1–6, β1–3, γ1–3, δ, ε, θ, π and ρ1–3) [30,31]. The typical GABA receptors consists of five subunits, with general subunit stoichiometry of 2α, 2β, and 1γ [32-34], arranged pseudo-symmetrically around central ion pore [35]. Bohlhalter et al. [36] discovered that within the spinal cord, the GABAA receptor subunits α3, β2,3 and γ2 exhibited widespread expression, contrasting with the more confined lamina-specific distribution of the other three α subunits. Specially, α1 and α5 subunits were predominantly found in the intermediate region, while α2 subunits prevailed in the superficial layers of the dorsal horn and in somatosensory and preganglionic motoneurons. The unique expression patterns of GABAA receptor subtypes across laminar layers in the spinal cord suggests that the diversity of GABAA receptors plays a role in regulating nociception, sensory input, and motor control at the segmental level [36].
Several compounds have been identified and are claimed to modulate selectively individual GABA receptor subtypes. These compounds can modulate GABA-induced currents or directly activate GABA receptors by binding to allosteric sites on GABA receptors or directly interacting with the GABA binding site, respectively [37]. Benzodiazepines, anesthetics, neurosteroids, picrotoxin, and many other molecules can allosterically modulate GABA receptors [38]. Numerous studies have highlighted the possible effects of flavonoids on the CNS via modulation of GABA receptor activity [8,39]. For example, flavonoids such as apigenin, 6-methylapigenin, dinatin, skrofulein, cirsilineol, hispidulin, and naringenin show affinity for GABA-benzodiazepines sites [11,40-42]. The 2ʹ-hydroxyl group of flavonoids is reported as relevant for its benzodiazepine binding affinity. Wang et al. [43] suggested that flavonoid baicalin-induced responses on SG neurons were mediated via the activation of the benzodiazepines site of GABA receptors [43,44]. However, some studies also report that flavonoids such as quercetin and apigenin affinity for the GABA receptor is independent of the benzodiazepine site [45,46]. Such benzodiazepine-independent mechanism is reported in responses mediated by α1β1γ2s GABA on Xenopus oocytes [45] and α1β2γ2 GABA on HEK293 cells [46].
Naringenin, a citrus flavonoid, exhibits various effects on the CNS and can bind to GABA receptors, exerting anxiolytic effects due to its ability to permeate the blood-brain barrier [47-49]. In this study, we found that naringenin enhanced GABA-receptor-mediated currents and AUC in the majority of neurons tested. This enhancement of GABA receptor activities can be explained by naringenin’s allosteric modulation of GABA receptors as flavonoids are known to interact with benzodiazepine binding sites on GABA receptors [8]. In addition, radioligand binding assay reveals that substances like (S) naringenin, glabrol, and 8-lavandulyl- flavanones act on the GABA receptors’ benzodiazepine site [50]. Besides flavonoid naringenin, essential oil linalool [51], terpene borneol [52], and biphenyl honokiol [53] possess the potential to influence the GABA receptor-mediated action on SG neurons of Vc. Notably, 6-hydroxyflavone, a flavone, shows diverse responses towards GABA, acting as a subtype-selective partial positive allosteric modulator at the flumazenil-sensitive benzodiazepine site and flumazenil-sensitive partial inverse agonist or negative modulator at α1β3γ2, α2β3γ2, and α5β3γ2 but not at α3β3γ2 receptor subunits [8]. The sensitivity and response to GABA can vary depending on subunit combinations, as seen with antiepileptic drugs like carbamazepine and phenytoin, which modulate GABAARs in a subunit-dependent manner. Both carbamazepine and phenytoin enhance GABA-induced currents in cultured rat cortical neurons and HEK293 cells transiently expressing the GABAAR α1β2γ2 subtype, however; GABAAR α1β2 subtype remained unaffected, and minimal effects was observed for the α3β2γ2 and α5β2γ3 receptor isoforms [54].
Interestingly, naringenin showed varied effects on GABA response depending on sex. For most female SG neurons, naringenin only enhanced GABA responses, whereas both enhancing and inhibiting effects were observed in males. Benzodiazepines, GABA receptor allosteric modulators, binding pocket have been identified in the cleft between the α1 and γ2 subunits of the GABA receptors [55]. Ravizza et al. [56] reported higher expression of α1 subunit mRNA in substantia nigra pars reticulate regions of females compared to the male of the same age groups (PND 15 and PND 30). Based on this evidence, we may speculate that naringenin exhibited varied effects on GABA receptor action across sex might be dependent on GABA receptor subunits composition. However, no findings are reported for the sex-dependent variation in GABA receptor subunits in the SG region and require further research.
The results of this study imply that naringenin’s responsiveness to the GABA receptor differs by sex, and these findings suggest that pain control mechanisms may differ depending on sex. This variation might be dependent on the level of expression of GABA receptor subunits. The GABA receptors' pharmacology, pattern of expression in the brain, and biophysical properties including their affinity for GABA are all influenced by the subunit composition [57]. To conclude, considering that SG neurons of the Vc as a principal region for modulation of orofacial nociception, the sex-dependent effect of naringenin on GABA receptors might contribute to sex-dependent orofacial pain modulation, at least in part.
REFERENCES
1. Bereiter DA, Hirata H, Hu JW. 2000; Trigeminal subnucleus caudalis: beyond homologies with the spinal dorsal horn. Pain. 88:221–224. DOI: 10.1016/S0304-3959(00)00434-6. PMID: 11068108.
2. Tsai CM, Chiang CY, Yu XM, Sessle BJ. 1999; Involvement of trigeminal subnucleus caudalis (medullary dorsal horn) in craniofacial nociceptive reflex activity. Pain. 81:115–128. DOI: 10.1016/S0304-3959(99)00009-3. PMID: 10353499.
3. Sheikh NK, Dua A. 2023. Neuroanatomy, substantia gelatinosa. StatPearls Publishing;DOI: 10.1007/springerreference_183581.
4. Cervero F, Iggo A. 1980; The substantia gelatinosa of the spinal cord: a critical review. Brain. 103:717–772. DOI: 10.1093/brain/103.4.717. PMID: 7437888.
5. Ren K, Dubner R. 2011; The role of trigeminal interpolaris-caudalis transition zone in persistent orofacial pain. Int Rev Neurobiol. 97:207–225. DOI: 10.1016/B978-0-12-385198-7.00008-4. PMID: 21708312. PMCID: PMC3257052.
6. Ghurye S, McMillan R. 2017; Orofacial pain - an update on diagnosis and management. Br Dent J. 223:639–647. DOI: 10.1038/sj.bdj.2017.879. PMID: 29074941.
7. Christidis N, Al-Moraissi EA. 2022; Editorial: orofacial pain of muscular origin-from pathophysiology to treatment. Front Oral Health. 2:825490. DOI: 10.3389/froh.2021.825490. PMID: 35098212. PMCID: PMC8794806. PMID: 2ccb4fe629be4cedbd1dbe5a0cd9e348.
8. Hanrahan JR, Chebib M, Johnston GA. 2011; Flavonoid modulation of GABA(A) receptors. Br J Pharmacol. 163:234–245. DOI: 10.1111/j.1476-5381.2011.01228.x. PMID: 21244373. PMCID: PMC3087128.
9. Enna SJ, McCarson KE. 2006; The role of GABA in the mediation and perception of pain. Adv Pharmacol. 54:1–27. DOI: 10.1016/S1054-3589(06)54001-3. PMID: 17175808.
10. Wang C, Hao H, He K, An Y, Pu Z, Gamper N, Zhang H, Du X. 2021; Neuropathic injury-induced plasticity of GABAergic system in peripheral sensory ganglia. Front Pharmacol. 12:702218. DOI: 10.3389/fphar.2021.702218. PMID: 34385921. PMCID: PMC8354334. PMID: 6616cc7a84fa467f8ebe0c9ac012381f.
11. Jäger AK, Almqvist JP, Vangsøe SAK, Stafford GI, Adsersen A, Van Staden J. 2007; Compounds from Mentha aquatica with affinity to the GABA-benzodiazepine receptor. S Afr J Bot. 73:518–521. DOI: 10.1016/j.sajb.2007.04.061.
12. Galati G, Moridani MY, Chan TS, O'Brien PJ. 2001; Peroxidative metabolism of apigenin and naringenin versus luteolin and quercetin: glutathione oxidation and conjugation. Free Radic Biol Med. 30:370–382. DOI: 10.1016/S0891-5849(00)00481-0. PMID: 11182292.
13. Hsu HT, Tseng YT, Lo YC, Wu SN. 2014; Ability of naringenin, a bioflavonoid, to activate M-type potassium current in motor neuron-like cells and to increase BKCa-channel activity in HEK293T cells transfected with α-hSlo subunit. BMC Neurosci. 15:135. DOI: 10.1186/s12868-014-0135-1. PMID: 25539574. PMCID: PMC4288500.
14. Palma-Duran SA, Caire-Juvera G, Robles-Burgeño Mdel R, Ortega-Vélez MI, Gutiérrez-Coronado Mde L, Almada Mdel C, Chávez-Suárez K, Campa-Siqueiros M, Grajeda-Cota P, Saucedo-Tamayo Mdel S, Valenzuela-Quintanar AI. 2015; Serum levels of phytoestrogens as biomarkers of intake in Mexican women. Int J Food Sci Nutr. 66:819–825. DOI: 10.3109/09637486.2015.1092019. PMID: 26417700.
15. Cavia-Saiz M, Busto MD, Pilar-Izquierdo MC, Ortega N, Perez-Mateos M, Muñiz P. 2010; Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: a comparative study. J Sci Food Agric. 90:1238–1244. DOI: 10.1002/jsfa.3959. PMID: 20394007.
16. Yang Z, Kuboyama T, Tohda C. 2017; A systematic strategy for discovering a therapeutic drug for Alzheimer's disease and its target molecule. Front Pharmacol. 8:340. DOI: 10.3389/fphar.2017.00340. PMID: 28674493. PMCID: PMC5474478. PMID: 5f3502fc5dd54124bf34c68fc45d4f67.
17. Manchope MF, Casagrande R, Verri WA Jr. 2017; Naringenin: an analgesic and anti-inflammatory citrus flavanone. Oncotarget. 8:3766–3767. DOI: 10.18632/oncotarget.14084. PMID: 28030851. PMCID: PMC5354790.
18. Straub I, Mohr F, Stab J, Konrad M, Philipp SE, Oberwinkler J, Schaefer M. 2013; Citrus fruit and fabacea secondary metabolites potently and selectively block TRPM3. Br J Pharmacol. 168:1835–1850. DOI: 10.1111/bph.12076. PMID: 23190005. PMCID: PMC3623054.
19. Pinho-Ribeiro FA, Zarpelon AC, Fattori V, Manchope MF, Mizokami SS, Casagrande R, Verri WA Jr. 2016; Naringenin reduces inflammatory pain in mice. Neuropharmacology. 105:508–519. DOI: 10.1016/j.neuropharm.2016.02.019. PMID: 26907804.
20. Park SA, Yang EJ, Han SK, Park SJ. 2012; Age-related changes in the effects of 5-hydroxytryptamine on substantia gelatinosa neurons of the trigeminal subnucleus caudalis. Neurosci Lett. 510:78–81. DOI: 10.1016/j.neulet.2011.12.069. PMID: 22260792.
21. Fitzgerald M, Koltzenburg M. 1986; The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat spinal cord. Brain Res. 389:261–270. DOI: 10.1016/0165-3806(86)90194-X. PMID: 3948011.
22. Chaudhary CL, Lim D, Chaudhary P, Guragain D, Awasthi BP, Park HD, Kim JA, Jeong BS. 2022; 6-Amino-2,4,5-trimethylpyridin-3-ol and 2-amino-4,6-dimethylpyrimidin-5-ol derivatives as selective fibroblast growth factor receptor 4 inhibitors: design, synthesis, molecular docking, and anti-hepatocellular carcinoma efficacy evaluation. J Enzyme Inhib Med Chem. 37:844–856. DOI: 10.1080/14756366.2022.2048378. PMID: 35296193. PMCID: PMC8933034. PMID: 2eb5573b9d544e4da8e77afce9c0885c.
23. Neher E, Sakmann B. 1992; The patch clamp technique. Sci Am. 266:44–51. DOI: 10.1038/scientificamerican0392-44. PMID: 1374932.
24. Todd AJ. 2010; Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 11:823–836. DOI: 10.1038/nrn2947. PMID: 21068766. PMCID: PMC3277941.
25. Zhu M, Yan Y, Cao X, Zeng F, Xu G, Shen W, Li F, Luo L, Wang Z, Zhang Y, Zhang X, Zhang D, Liu T. 2021; Electrophysiological and morphological features of rebound depolarization characterized interneurons in rat superficial spinal dorsal horn. Front Cell Neurosci. 15:736879. DOI: 10.3389/fncel.2021.736879. PMID: 34621158. PMCID: PMC8490703. PMID: 83563b199c0d456ba1bbb859d54cdfcc.
26. Polgár E, Hughes DI, Riddell JS, Maxwell DJ, Puskár Z, Todd AJ. 2003; Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 104:229–239. DOI: 10.1016/S0304-3959(03)00011-3. PMID: 12855333.
27. Kim D, Kim B. 2022; Anatomical and functional differences in the sex-shared neurons of the nematode C. elegans. Front Neuroanat. 16:906090. DOI: 10.3389/fnana.2022.906090. PMID: 35601998. PMCID: PMC9121059. PMID: 87a194909b5a40c48f66ec097bc4fc90.
28. Chudomel O, Herman H, Nair K, Moshé SL, Galanopoulou AS. 2009; Age- and gender-related differences in GABAA receptor-mediated postsynaptic currents in GABAergic neurons of the substantia nigra reticulata in the rat. Neuroscience. 163:155–167. DOI: 10.1016/j.neuroscience.2009.06.025. PMID: 19531372. PMCID: PMC2729356.
29. Jiang CY, Fujita T, Kumamoto E. 2016; Developmental change and sexual difference in synaptic modulation produced by oxytocin in rat substantia gelatinosa neurons. Biochem Biophys Rep. 7:206–213. DOI: 10.1016/j.bbrep.2016.06.011. PMID: 28955908. PMCID: PMC5613344.
30. McCarthy MM, Auger AP, Perrot-Sinal TS. 2002; Getting excited about GABA and sex differences in the brain. Trends Neurosci. 25:307–312. DOI: 10.1016/S0166-2236(02)02182-3. PMID: 12086749.
31. Olsen RW, Sieghart W. 2008; International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 60:243–260. DOI: 10.1124/pr.108.00505. PMID: 18790874. PMCID: PMC2847512.
32. Jansen M, Rabe H, Strehle A, Dieler S, Debus F, Dannhardt G, Akabas MH, Lüddens H. 2008; Synthesis of GABAA receptor agonists and evaluation of their alpha-subunit selectivity and orientation in the GABA binding site. J Med Chem. 51:4430–4448. DOI: 10.1021/jm701562x. PMID: 18651727. PMCID: PMC2566937.
33. Nutt DJ, Malizia AL. 2001; New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br J Psychiatry. 179:390–396. DOI: 10.1192/bjp.179.5.390. PMID: 11689393.
34. Sigel E. 2002; Mapping of the benzodiazepine recognition site on GABA(A) receptors. Curr Top Med Chem. 2:833–839. DOI: 10.2174/1568026023393444. PMID: 12171574.
35. Scott S, Aricescu AR. 2019; A structural perspective on GABAA receptor pharmacology. Curr Opin Struct Biol. 54:189–197. DOI: 10.1016/j.sbi.2019.03.023. PMID: 31129381.
36. Bohlhalter S, Weinmann O, Mohler H, Fritschy JM. 1996; Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. J Neurosci. 16:283–297. DOI: 10.1523/JNEUROSCI.16-01-00283.1996. PMID: 8613794. PMCID: PMC6578721.
37. Sieghart W, Savić MM. 2018; International Union of Basic and Clinical Pharmacology. CVI: GABAA receptor subtype- and function-selective ligands: key issues in translation to humans. Pharmacol Rev. 70:836–878. DOI: 10.1124/pr.117.014449. PMID: 30275042.
38. Korpi ER, Gründer G, Lüddens H. 2002; Drug interactions at GABA(A) receptors. Prog Neurobiol. 67:113–159. DOI: 10.1016/S0301-0082(02)00013-8. PMID: 12126658.
39. Youdim KA, Shukitt-Hale B, Joseph JA. 2004; Flavonoids and the brain: interactions at the blood-brain barrier and their physiological effects on the central nervous system. Free Radic Biol Med. 37:1683–1693. DOI: 10.1016/j.freeradbiomed.2004.08.002. PMID: 15528027.
40. Jäger AK, Saaby L. 2011; Flavonoids and the CNS. Molecules. 16:1471–1485. DOI: 10.3390/molecules16021471. PMID: 21311414. PMCID: PMC6259921. PMID: 9e58fef5e36944378eaede5d7db0d96f.
41. Liu Z, Silva J, Shao AS, Liang J, Wallner M, Shao XM, Li M, Olsen RW. 2021; Flavonoid compounds isolated from Tibetan herbs, binding to GABAA receptor with anxiolytic property. J Ethnopharmacol. 267:113630. DOI: 10.1016/j.jep.2020.113630. PMID: 33246118.
42. Stafford GI, Jäger AK, van Staden J. 2005; Activity of traditional South African sedative and potentially CNS-acting plants in the GABA-benzodiazepine receptor assay. J Ethnopharmacol. 100:210–215. DOI: 10.1016/j.jep.2005.04.004. PMID: 16054530.
43. Wang F, Xu Z, Ren L, Tsang SY, Xue H. 2008; GABA A receptor subtype selectivity underlying selective anxiolytic effect of baicalin. Neuropharmacology. 55:1231–1237. DOI: 10.1016/j.neuropharm.2008.07.040. PMID: 18723037.
44. Yin H, Bhattarai JP, Oh SM, Park SJ, Ahn DK, Han SK. 2016; Baicalin activates glycine and γ-aminobutyric acid receptors on substantia gelatinosa neurons of the trigeminal subsnucleus caudalis in juvenile mice. Am J Chin Med. 44:389–400. DOI: 10.1142/S0192415X16500221. PMID: 27080947.
45. Goutman JD, Waxemberg MD, Doñate-Oliver F, Pomata PE, Calvo DJ. 2003; Flavonoid modulation of ionic currents mediated by GABA(A) and GABA(C) receptors. Eur J Pharmacol. 461:79–87. DOI: 10.1016/S0014-2999(03)01309-8. PMID: 12586201.
46. Losi G, Puia G, Garzon G, de Vuono MC, Baraldi M. 2004; Apigenin modulates GABAergic and glutamatergic transmission in cultured cortical neurons. Eur J Pharmacol. 502:41–46. DOI: 10.1016/j.ejphar.2004.08.043. PMID: 15464088.
47. Youdim KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ. 2004; Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic Biol Med. 36:592–604. Erratum in: Free Radic Biol Med. 2004;36:1342. DOI: 10.1016/j.freeradbiomed.2004.03.003.
48. Medina JH, Viola H, Wolfman C, Marder M, Wasowski C, Calvo D, Paladini AC. 1998; Neuroactive flavonoids: new ligands for the Benzodiazepine receptors. Phytomedicine. 5:235–243. DOI: 10.1016/S0944-7113(98)80034-2. PMID: 23195847.
49. Paladini AC, Marder M, Viola H, Wolfman C, Wasowski C, Medina JH. 1999; Flavonoids and the central nervous system: from forgotten factors to potent anxiolytic compounds. J Pharm Pharmacol. 51:519–526. DOI: 10.1211/0022357991772790. PMID: 10411210.
50. Banerjee S, Manisha C, Murugan D, Justin A. El-Shemy HA, editor. 2022. Natural products altering GABAergic transmission. Natural medicinal plants. IntechOpen;p. 1–23. DOI: 10.5772/intechopen.99500.
51. Phuong TNT, Jang SH, Rijal S, Jung WK, Kim J, Park SJ, Han SK. 2021; GABA- and glycine-mimetic responses of linalool on the substantia gelatinosa of the trigeminal subnucleus caudalis in juvenile mice: pain management through linalool-mediated inhibitory neurotransmission. Am J Chin Med. 49:1437–1448. DOI: 10.1142/S0192415X21500671. PMID: 34247560.
52. Nguyen PTT, Jang SH, Rijal S, Park SJ, Han SK. 2020; Inhibitory actions of borneol on the substantia gelatinosa neurons of the trigeminal subnucleus caudalis in mice. Korean J Physiol Pharmacol. 24:433–440. DOI: 10.4196/kjpp.2020.24.5.433. PMID: 32830150. PMCID: PMC7445480.
53. Le HTN, Rijal S, Jang SH, Park SA, Park SJ, Jung W, Han SK. 2023; Inhibitory effects of honokiol on substantia gelatinosa neurons of the trigeminal subnucleus caudalis in juvenile mice. Neuroscience. 521:89–101. DOI: 10.1016/j.neuroscience.2023.04.022. PMID: 37142181.
54. Granger P, Biton B, Faure C, Vige X, Depoortere H, Graham D, Langer SZ, Scatton B, Avenet P. 1995; Modulation of the gamma-aminobutyric acid type A receptor by the antiepileptic drugs carbamazepine and phenytoin. Mol Pharmacol. 47:1189–1196. PMID: 7603459.
55. Sigel E, Lüscher BP. 2011; A closer look at the high affinity benzodiazepine binding site on GABAA receptors. Curr Top Med Chem. 11:241–246. DOI: 10.2174/156802611794863562. PMID: 21189125.
56. Ravizza T, Friedman LK, Moshé SL, Velísková J. 2003; Sex differences in GABA(A)ergic system in rat substantia nigra pars reticulata. Int J Dev Neurosci. 21:245–254. DOI: 10.1016/S0736-5748(03)00069-8. PMID: 12850057.
57. Goetz T, Arslan A, Wisden W, Wulff P. 2007; GABA(A) receptors: structure and function in the basal ganglia. Prog Brain Res. 160:21–41. DOI: 10.1016/S0079-6123(06)60003-4. PMID: 17499107.
Fig. 2
Effects of naringenin and GABA-mediated responses of substantia gelatinosa (SG) neurons under a voltage clamp mode.
(A) A representative trace showing the effect of naringenin in a dose-dependent manner (1 μM, 10 μM, 100 μM, 1 mM, and 2 mM, n = 10). (B) A histogram illustrates a weak inward current observed above 1 mM concentration of naringenin. (C, D) A representative trace and histogram show no discernible effect of 0.5 mM naringenin on membrane current (n = 3). (E–H) Representative traces and histograms showing no desensitization of slow (E, G, n = 15) and fast (F, H, n = 6) GABA responses upon repeated application to SG neurons. (I) A histogram demonstrates no significant differences in the response mediated by GABA upon repeated application (total n = 21, paired t-test, p > 0.05, ns: not significant).

Fig. 3
Effects of naringenin on GABA-mediated responses of substantia gelatinosa (SG) neurons under a voltage clamp mode.
(A, D) Representative current traces illustrate the effect of naringenin on 30 μM GABA-induced inward slow and fast responses, respectively. (B, C) Histograms display a significant enhancement in the mean relative amplitude and area under curve (AUC) of GABA-induced slow responses by 0.5 mM naringenin (n = 12), respectively. (E, F) Histograms showing significant suppression in the mean relative amplitude and AUC of GABA-induced fast responses by 0.5 mM naringenin (n = 7), respectively (paired t-test, *p < 0.05, **p < 0.01, ***p < 0.001).
Fig. 4
Dose-dependent effect of naringenin on GABA-mediated slow and fast responses of substantia gelatinosa (SG) neurons.
(A, B) Representative traces showing dose-dependent effect of naringenin (1 μM, 10 μM, 100 μM, and 1 mM) on 30 μM GABA-induced slow and fast inward current, respectively. (C) A histogram compares the increasing tendency in the mean relative amplitude of 30 μM GABA-mediated slow responses in the presence of various concentrations of naringenin (n = 4). (D) A histogram compares the decreasing tendency in the mean relative amplitude of 30 μM GABA-mediated fast responses in the presence of various concentrations of naringenin (n = 5) (*p < 0.05, **p < 0.01, one-way ANOVA post-hoc Scheffe test).
Fig. 5
Effects of naringenin on GABA-mediated responses of substantia gelatinosa (SG) neurons under a current clamp mode.
(A) Representative current traces demonstrate the effects of naringenin on 30 μM GABA-induced depolarized responses. (B) A histogram indicates no significant difference in GABA-induced depolarization in the presence of 0.5 mM naringenin (n = 5). (C) A histogram displays a significant increase in the relative area under the curve (AUC) of GABA-induced depolarization in the presence of 0.5 mM naringenin (n = 5) (Paired t-test, nsp > 0.05, *p < 0.05, ns: not significant, respectively). RMP, resting membrane potential; Cc, current clamp.
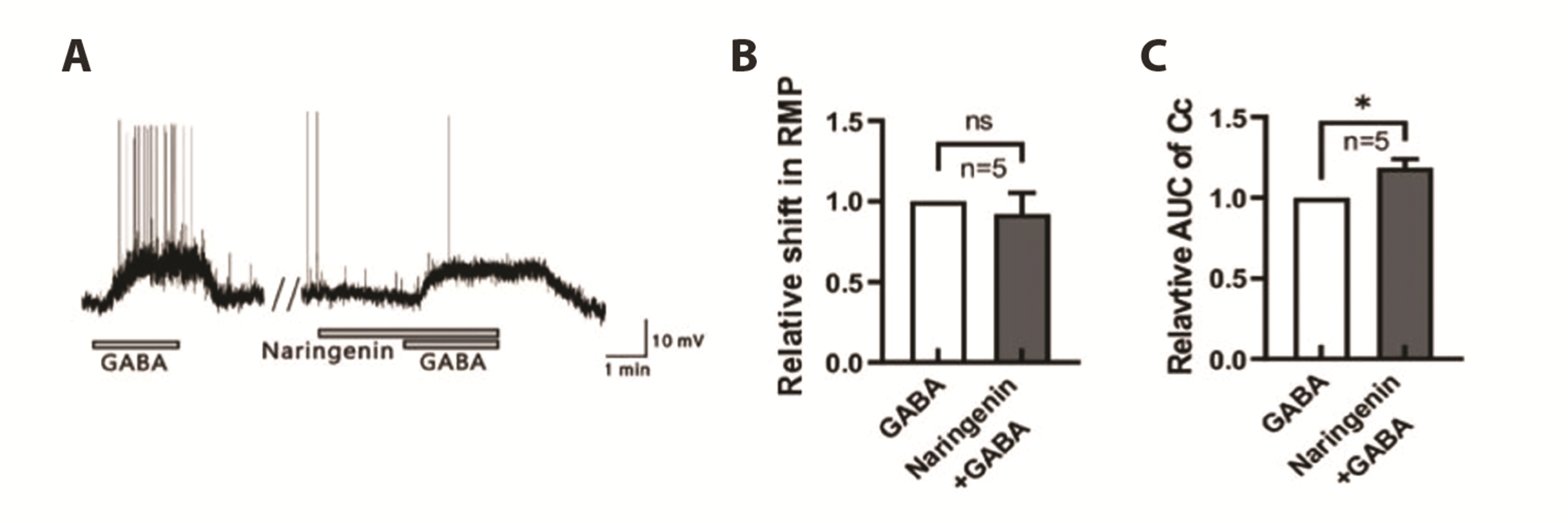
Fig. 6
Histogram depicting the varied nature of GABA responses in male and female juvenile substantia gelatinosa (SG) neurons.
(A) Histogram illustrating the percentage of varied GABA response nature across male and female juvenile SG neurons. (B, C) Histogram indicating the percentage of varied effects of naringenin on GABA-mediated inward current and area under the curve (AUC) across male and female, rerspectively (chi-square test, *p < 0.05).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download