INTRODUCTION
Transcatheter aortic valve implantation (TAVI) has changed the management strategy for patients with severe aortic stenosis (AS) since its introduction in the early 2000s. Based on randomized controlled trials
123 demonstrating TAVI as a treatment of choice regardless of operative risk, the 2020 American College of Cardiology/American Heart Association guidelines recommended the choice between TAVI and surgical aortic valve replacement (SAVR) only based on the patient’s age and life expectancy.
4 However, recent practice has led to a rapid increase in TAVI utilization across a wide range of patients, regardless of age.
56 Currently, in Western countries, TAVI has become the predominant choice of treatment for severe AS in all spectrums of patients.
78910
In Korea, TAVI was first introduced in 2010.
11 Initially, the volume of TAVI was very low, and the patient’s burden of expenses was high because it was not covered by insurance from the National Health Insurance Service (NHIS). At that time, TAVI procedures were not monitored by the Health Insurance Review and Assessment Service (HIRA), a public institution that plays a role between healthcare providers and the NHIS, and the NHIS database regarding TAVI was not yet available. However, since June 2015 when the NHIS began to include TAVI in its insurance coverage, the TAVI database was established and monitored by the HIRA. The volume of TAVI has grown sharply in Korea since then.
A previous study reported that the proportion of low-risk patients occupied up to 85% of the population requiring aortic valve intervention.
12 In anticipation of the increased practice of TAVI in lower surgical risk cohorts, the volume of TAVI in Korea is expected to show a rapid increase over the next decade. In these circumstances, the contemporary outcomes of SAVR in Korea need to be evaluated as a potential comparator to those of TAVI in future studies.
The aim of this study was to evaluate contemporary outcomes of SAVR in a multicenter study in Korea as a potential control group for upcoming TAVI studies.
METHODS
Patient selection
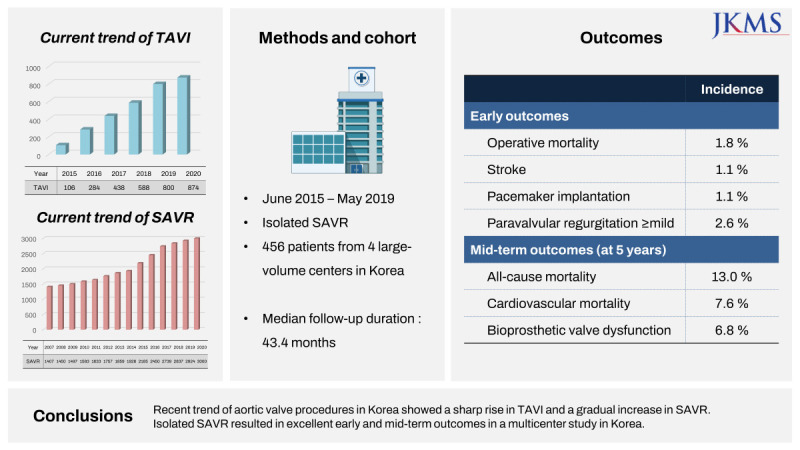
The study population in this analysis was selected with the intention: 1) to perform interim analysis related to the national health insurance policy and 2) to construct a control SAVR group to the TAVI population in a potential comparative analysis. Because TAVI has become a part of national health insurance coverage since June 2015, we selected SAVR patients who received the operation within 5 years of June 2015. We sampled patients from 4 large-volume centers who received isolated SAVR, expecting the sample population to be a potential comparator to isolated transfemoral TAVI. The centers whose volume of isolated SAVR using bioprostheses was over 20 cases annually and who agreed to provide their institutional data of 5 years for isolated SAVR participated in this study.
Inclusion criteria were: 1) patients who underwent isolated SAVR from June 2015 to May 2019 and 2) aged ≥ 19. Exclusion criteria were: 1) other concomitant cardiac procedures (e.g., other valve surgery, coronary artery bypass grafting, aorta surgery, or arrhythmia surgery), 2) SAVR with a mechanical valve, 3) involvement of annulus enlargement procedure, and 4) infective endocarditis. A total of 456 patients from 4 institutions were included in this analysis.
Evaluation of clinical outcomes
Periprocedural mortality was defined as any death within 30 days after the index procedure or any death during the index hospitalization. After discharge, all patients underwent regular postoperative follow-ups through the outpatient clinic in each institution. For the patients who were lost to follow-up, their survival or mortality date was confirmed by the death certificates from Statistics Korea, a central organization for statistics under the Ministry of Strategy and Finance. The clinical follow-ups ended in June 2022. The median follow-up duration was 43.4 months (interquartile range [IQR], 33.8–59.6).
Cardiovascular mortality was defined as death meeting one of the following criteria: 1) related to heart failure, cardiogenic shock, bioprosthetic valve dysfunction, myocardial infarction, stroke, thromboembolism, bleeding, tamponade, vascular complication, arrhythmia or conduction system disturbances, cardiovascular infection (e.g., mediastinitis, endocarditis), or other clear cardiovascular cause, 2) intraprocedural death, 3) sudden death, and 4) death of unknown cause. Bioprosthetic valve dysfunction was defined as structural valve deterioration, nonstructural valve dysfunction, thrombosis, and endocarditis.
13 Stroke was defined as overt central nervous system injury (NeuroARC Type 1) according to the guidelines.
13
Evaluation of hemodynamic outcomes
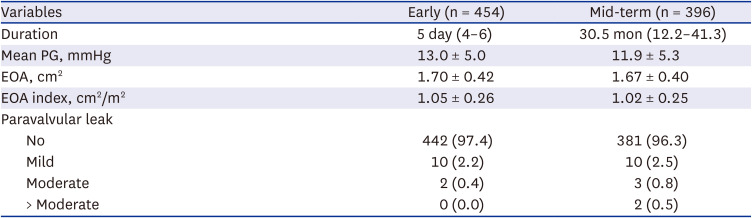
Early postoperative and follow-up echocardiograms were regularly performed regardless of the patient’s clinical status or symptoms in each institution. Early postoperative echocardiography was obtained at median postoperative Day 5 (IQR, 4–6) from 454 patients (99.6%). Mid-term follow-up echocardiography was collected at a median of 30.5 months (IQR, 12.2–41.3) after surgery from 396 patients (86.8%).
The changes in hemodynamic properties were analyzed with the mean pressure gradient (PG) across the prosthetic valve, effective orifice area (EOA) and EOA index. The degrees of paravalvular regurgitation were also evaluated in early and mid-term follow-up echocardiograms.
Statistical analysis
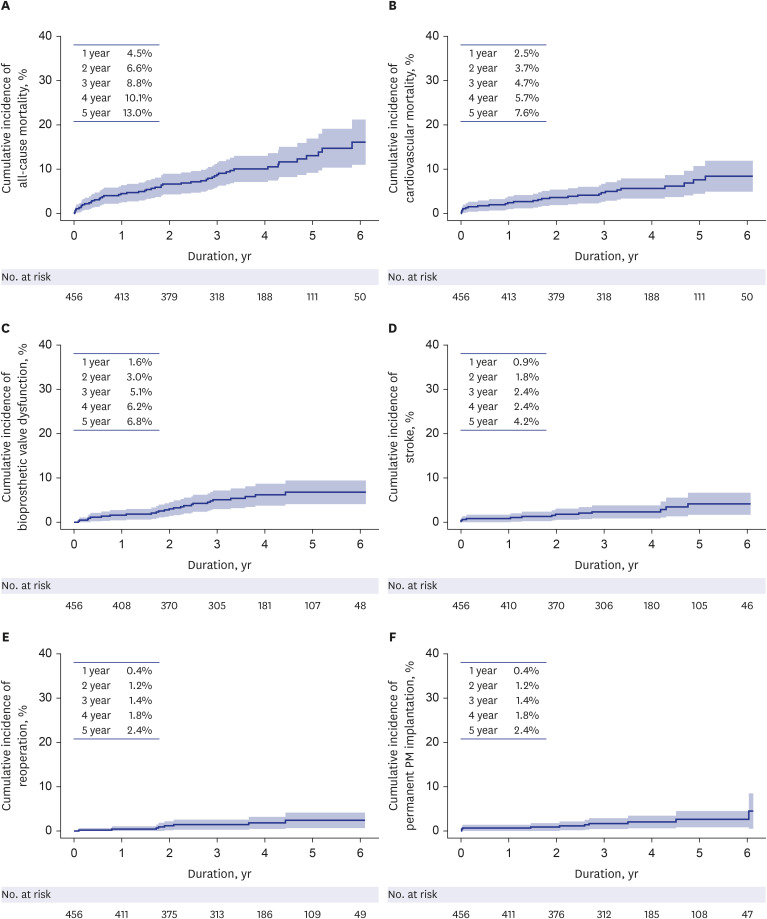
Statistical analysis was conducted with SPSS software (IBM, Armonk, NY, USA) and SAS software (SAS Institute, Cary, NC, USA). Continuous variables are presented as the mean ± standard deviation for normally distributed data or median with IQR for data that were not normally distributed, whereas categorical variables are presented as the number and percentage of subjects. All-cause mortality was estimated using the Kaplan-Meier method. For the analysis of cardiovascular mortality, bioprosthetic valve dysfunction, stroke, reoperation, and permanent pacemaker implantation, cumulative incidence curves were estimated while considering competing events. Noncardiovascular mortality was considered a competing event for cardiovascular mortality, and all-cause mortality was considered a competing event for bioprosthetic valve dysfunction, stroke, reoperation, and permanent pacemaker implantation.
Ethics statement
The study protocol was reviewed by the Institutional Review Board and approved as a minimal-risk retrospective study in each institution (Approval Number: 2023-02-126 in Samsung Medical Center, B-2210-786-402 in Seoul National University Bundang Hospital, H-2110-075-1262 in Seoul National University Hospital, and 4-2023-0079 in Severance Hospital) that did not require individual consent based on the institutional guidelines for waiving consent.
DISCUSSION
The present study demonstrated 2 main findings. First, the early and mid-term clinical outcomes of isolated SAVR in the participating centers from Korea were as excellent as those in high-volume centers worldwide. Second, early and mid-term hemodynamic outcomes, including the incidence of paravalvular regurgitation, of isolated SAVR were also excellent.
As supplementary data for this study, the current trends of TAVI and SAVR were simultaneously analyzed using the NHIS database. According to the NHIS database, the number of patients who underwent SAVR gradually increased, from 1,407 patients in 2007 to 3,000 patients in 2020 (
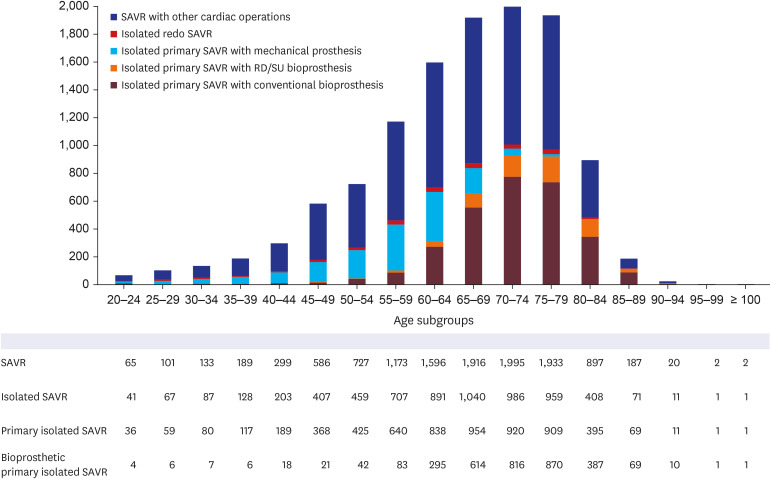
Fig. 2). When the patient cohort was confined to those who received SAVR during the last 5 years, 11,916 patients underwent SAVR from June 2015 to May 2019 in Korea. Among them, 45.2% of the patients (5,390 out of 11,916) underwent isolated SAVR, 95.2% of the patients (5,132 out of 5,390) underwent primary isolated SAVR, and 70.4% of the patients (3,612 out of 5,132) underwent bioprosthetic primary isolated SAVR. When the patient cohort was divided into the age subgroups with an interval of 5 years, bioprosthetic primary isolated SAVR was most frequently performed in the 70–74 and 75–79 age subgroups, accounting for 25.7% and 25.6% of the cohort, respectively (
Supplementary Table 3). SAVR with concomitant cardiac operations was performed in 54.8% of the entire cohort (6,526 out of 11,916), primary SAVR with concomitant cardiac operations was performed in 92.9% of the patients (6,065 out of 6,526), and bioprosthetic primary SAVR with concomitant cardiac operations were performed in 53.6% of the patients (3,249 out of 6,065). Bioprosthetic primary SAVR with concomitant cardiac operations was most frequently performed in the age subgroups of 75–79 and 70–74, occupying 26.8% and 25.1% of the cohort, respectively (
Fig. 3,
Supplementary Table 4).
Fig. 2
Annual trend of SAVR from 2007 to 2020 in Korea.
SAVR = surgical aortic valve replacement.
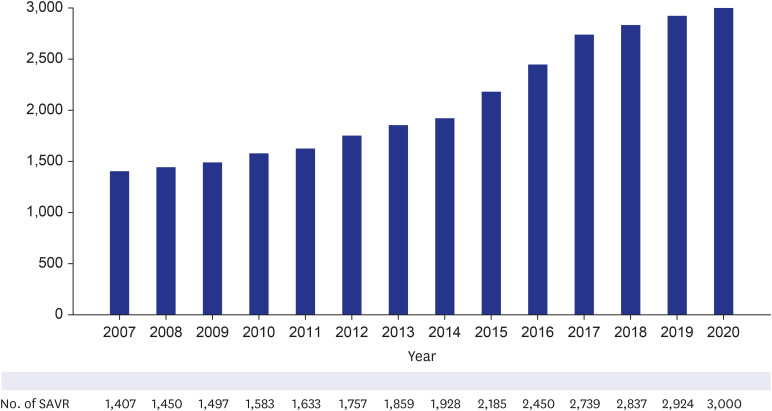
Fig. 3
Number of patients for each age subgroup classified by the type of SAVR from June 2015 to May 2020. SAVR was classified into isolated SAVR and SAVR with concomitant cardiac operations; isolated SAVR was classified into primary SAVR and redo SAVR; primary SAVR was classified into bioprosthetic SAVR and mechanical SAVR; and bioprosthetic SAVR was classified into conventional SAVR and RD/SU AVR.
RD = rapid-deployment, SAVR = surgical aortic valve replacement, SU = sutureless.
The number of patients who underwent TAVI rapidly increased since 2015, the year in which TAVI was authorized to be covered by the NHIS and was incorporated into the NHIS database (
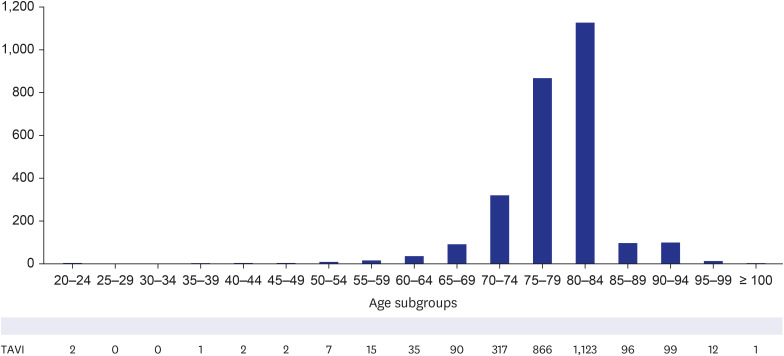
Fig. 4). During the 5 years after authorization, 3,249 patients underwent TAVI in Korea. Female patients accounted for 44.9% of the patients, and 98.5% (3,022 out of 3,249) of the procedures were performed via the transfemoral approach. When the patient cohort was divided into age subgroups with an interval of 5 years, TAVI was most frequently performed in the age subgroups of 75–79 and 70–74, occupying 26.8% and 25.1% of the cohort, respectively (
Fig. 5,
Supplementary Table 5).
Fig. 4
Annual trend of TAVI from 2015 to 2020 in Korea.
TAVI = transcatheter aortic valve implantation.
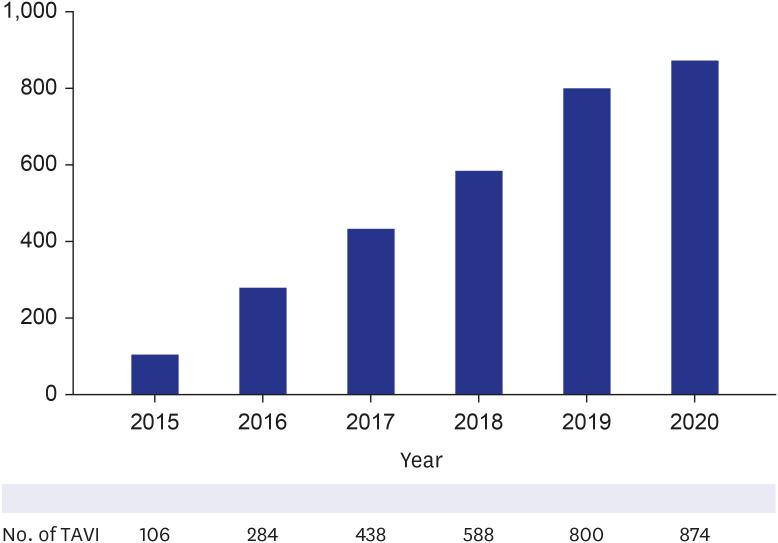
Fig. 5
Number of patients in each age subgroup who received TAVI from June 2015 to May 2020.
TAVI = transcatheter aortic valve implantation.
To our knowledge, these results from NHIS database presented the most recent trends regarding both TAVI and SAVR in Korea, reflecting the practice for the entire Korean population in the real world. Since 2015, the year TAVI was incorporated into national health insurance coverage,
14 the volume of TAVI has increased sharply, and it is now performed in over 1,000 cases annually. The annual numbers of TAVI procedures in Korea are expected to rise consistently in the following years, similar to those in Western countries, which kept growing over 10 years and exceeded all forms of SAVR.
1015 Although increasing TAVI has not yet resulted in a significant decrease in SAVR volume in Korea, it would be of great influence on SAVR volume in the near future.
In this supplementary analysis, we also described the numbers of aortic valve procedures according to the age subgroups of 5-year intervals. The age distribution of patients who received TAVI was centered between 75 and 85 years of age. A previous report from another country showed a dramatic growth of TAVI in all age groups, especially < 65 years of age, and showed that there was near-equal utilization between TAVI and SAVR in these younger patients by 2021.
5 In Korea, TAVI utilization was not yet predominant in patients < 75 years of age. However, based on the trend of TAVI expansion, it is anticipated that TAVI utilization will be extended to the entire population regardless of age and surgical risks. These predictions have implications that lifetime management strategies in young patients with severe AS are needed, including issues related to lifetime coronary access, valve durability, and future subsequent reintervention.
When TAVI was first incorporated into insurance coverage in Korea, the copay of the patients was initially 80% of the cost. At that time, the official announcement by the Ministry of Health and Welfare of Korea documented the indications for TAVI to be severe AS with New York Heart Association class ≥ 2 and high/prohibitive risk patients with STS score > 8% and predicted operative mortality ≥ 15%, while the contraindications were expected lifespan ≤ 1 year, no chance for improvement in quality of life, or the requirement of other concomitant valvular surgery. Despite the official announcement from the government that regulated performing TAVI for low/intermediate risk patients, real-world practices did not comply with the official regulations. TAVI was widely performed for low- and intermediate-risk patients based on the updated guidelines, and excellent outcomes following TAVI were reported as well.
16
In this circumstance, much attention has focused on comparing the outcomes in low-risk patients between TAVI and SAVR. Consecutive multicenter randomized trials have been and are being performed in patients with lower surgical risk, and are reporting equivalent and even superior early outcomes of TAVI.
317 However, it is noteworthy that there has been criticism that the methodology of these industry-sponsored trials was largely designed to favor TAVI through strict patient selection criteria.
1819 The advocates for SAVR also insist that outcome data about SAVR coming from industry-driven randomized trials seem to be suboptimal.
16 To offer some balance to this argument, we designed this study to establish a potential control group to the TAVI group, thus enrolling the patients who underwent isolated primary SAVR, excluding concomitant cardiac procedures and endocarditis patients. In addition, based on the evidence that higher procedure volumes result in better outcomes,
2021 we recruited high-volume SAVR centers in Korea at which excellent outcomes can be achieved following SAVR. Our study provides a real-world experience of surgical results to improve the understanding of the risks of surgery and decision-making in a multidisciplinary team setting with a heart team.
The outcomes from the current study are consistent with those of previous large international studies. In-hospital mortality for isolated SAVR was 1.8% in this study. It was very close to the outcome from the report that evaluated 234,556 patients from the STS database from 2013 to 2018 and demonstrated that operative mortality was 2.1% in the overall cohort and 1.5% in institutions performing > 100 SAVRs per year.
22 A recent analysis of the Japanese Cardiovascular Surgery database that assessed the outcomes of patients undergoing SAVR over 8 years demonstrated a similar in-hospital mortality of 2%.
23 A recent study from a multicenter European registry with 1,192 patients presented 30-day mortality of 1.5%.
16 Given that the present study was performed in high volume centers, early mortality of 1.8% might be not satisfactory, and this result was also inferior to those of TAVI under the similar baseline conditions. However, it should also be taken into account that the patients undergoing SAVR with tissue valve usually have old age and many comorbidities which cause inevitable early mortality. In fact, among 8 early mortalities in this study, 4 patients were octogenarians, and the other 2 patients had multiple comorbidities including coronary disease, history of cerebrovascular event, and end-stage renal disease requiring renal replacement therapy. The incidence of stroke for isolated SAVR was 1.1% in this study, which was lower than those in previous studies reporting an incidence of 1.5–2.8%.
162024 The incidence of permanent pacemaker implantation was 1.1% (5 out of 456 patients) in this study, and was comparable to the previous studies reporting 0.07–5.3%.
202425 Of 5 patients who underwent pacemaker implantation, 4 were associated with sutureless or rapid-deployment valves.
Paravalvular regurgitation is another important issue regarding the outcomes of TAVI and SAVR. A recent meta-analysis, which focused on structural valve deterioration, demonstrated higher 1-year, 2–3 years, and 5-year rates of paravalvular regurgitation, moderate or severe aortic regurgitation, and reintervention in the TAVI group compared to the SAVR group.
26 A systemic review regarding TAVI valve durability concluded that long-term durability remains unclear, and limited by the currently available data.
27 It was also reported that paravalvular regurgitation, even with a mild grade, results in poor long-term outcomes.
28 Our results demonstrated a low incidence of paravalvular regurgitation with 2.6%, which was far superior to those in the TAVI series, and consequently, would produce better outcomes in the long term than the results of TAVI with significant paravalvular regurgitations.
Despite the current uncertainty on TAVI durability, it is clear that excellent outcomes of TAVI are impacting surgical practice. Attributed to the reliable option of future valve-in-valve procedures rather than a redo surgery and the increased durability of modern surgical tissue valves, bioprostheses are being implanted in patients of a younger age than in the past as a reasonable alternative to mechanical valve to reduce anticoagulation-associated risks.
29 In this study, 25.6% of the patients aged between 55 and 65 received bioprosthetic valves rather than mechanical valves.
This study has several limitations that should be recognized. First, this study was a retrospective observational study, and the sample size was relatively small, although we enrolled patients from 4 high-volume centers in Korea. To investigate the most recent outcomes of SAVR, we only included data from the last 5 years, which also limited the number of study population. Despite a multicenter design of this study, the sample size of this study was particularly small because we only included the patients who underwent SAVR ‘without any concomitant cardiac procedures’ and ‘without use of mechanical valve as an aortic valve substitute.’ The authors accepted the small cohort caused by the strict inclusion criteria in order to make this cohort close to the potential references for TAVI studies. Second, the follow-up duration of the study cohort was relatively short. Longer-term data would be required to discuss the outcomes of SAVR in comparison with TAVI. Long-term data from patients who underwent surgery in the 2000s are now available in many centers worldwide, and excellent outcomes have been reported in related studies. The patients enrolled in this study might demonstrate much improved long-term outcomes in future studies. Third, this study had a single-arm design and did not compare SAVR to TAVI. Well-designed comparative studies are necessary to establish sophisticated management guidelines for aortic valve disease. A future study exploring the comparative outcomes between TAVI and SAVR using NHIS database is also required. Fourth, the results from this study might not represent the standard isolated SAVR situation of Korea. Although this study was performed based on a multicenter cohort, the outcomes from our study might be deviated from the results from small-volume centers or from the entire centers of Korea.
The recent 5-year trend of aortic valve procedures in Korea showed a sharp rise in TAVI and a gradual increase in SAVR. Isolated SAVR using bioprostheses has been performed with excellent early and mid-term outcomes in a multicenter study in Korea.







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download