1. Kim KS, Kwon J, Kim K, Chie EK. Impact of resection margin distance on survival of pancreatic cancer: a systematic review and meta-analysis. Cancer Res Treat. 2017; 49(3):824–833. PMID:
27561314.

2. Rhee H, Park MS. The role of imaging in current treatment strategies for pancreatic adenocarcinoma. Korean J Radiol. 2021; 22(1):23–40. PMID:
32901458.

3. Zhang J, Zhang L, Li C, Yang C, Li L, Song S, et al. LOX-1 is a poor prognostic indicator and induces epithelial-mesenchymal transition and metastasis in pancreatic cancer patients. Cell Oncol (Dordr). 2018; 41(1):73–84. PMID:
29168159.

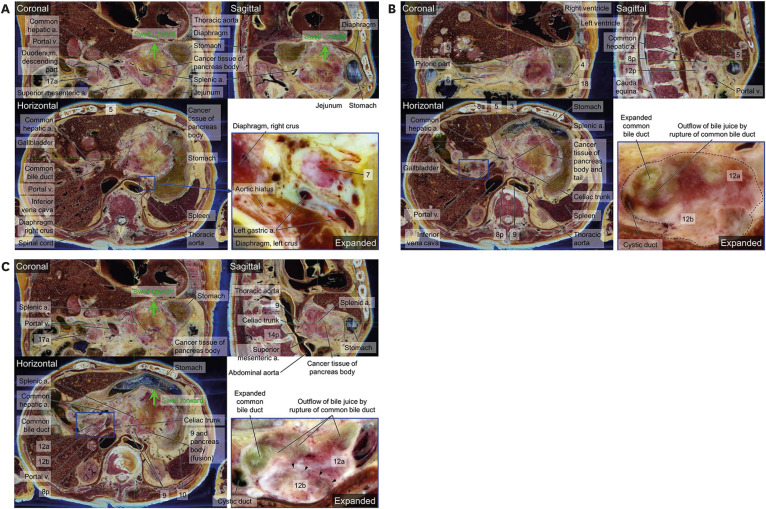
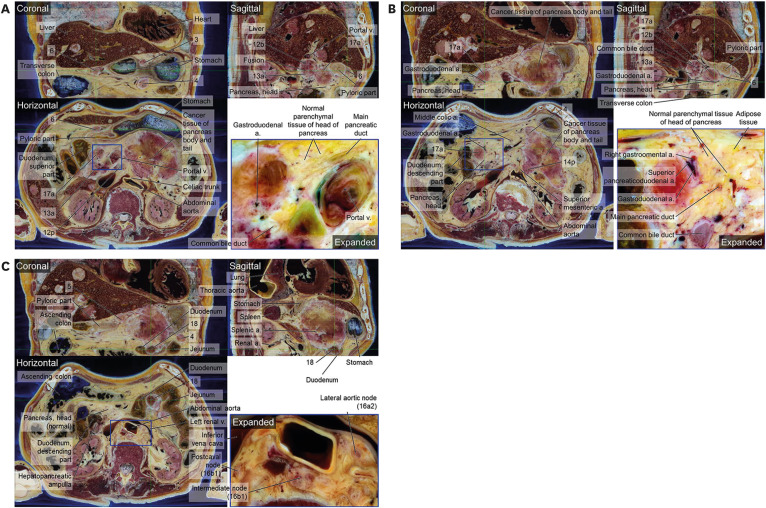
4. Isaji S, Murata Y, Kishiwada M. New Japanese Classification of Pancreatic Cancer. Neoptolemos JP, Urrutia R, Abbruzzese JL, Büchler MW, editors. Pancreatic Cancer. New York, NY, USA: Springer New York;2018. p. 1021–1037.
5. Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. Hoboken, NJ, USA: John Wiley & Sons;2011.
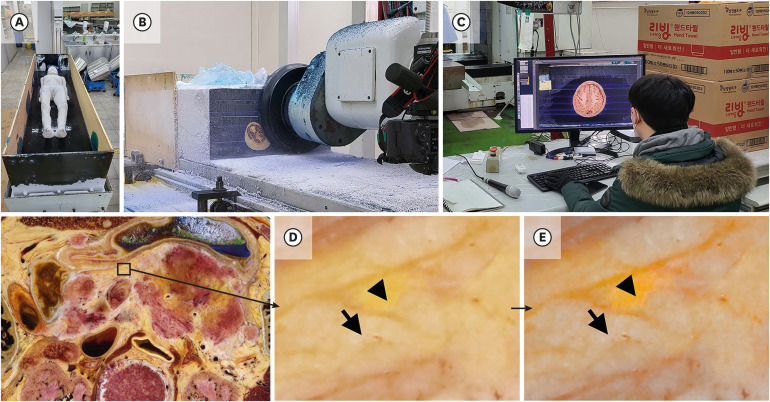
6. Park JS, Chung MS, Hwang SB, Lee YS, Har DH, Park HS. Visible Korean human: improved serially sectioned images of the entire body. IEEE Trans Med Imaging. 2005; 24(3):352–360. PMID:
15754985.

7. Park HS, Choi DH, Park JS. Improved sectioned images and surface models of the whole female body. Int J Morphol. 2015; 33(4):1323–1332.

8. Chung BS, Han M, Har D, Park JS. Advanced sectioned images of a cadaver head with voxel size of 0.04 mm. J Korean Med Sci. 2019; 34(34):e218. PMID:
31456382.

9. Shin DS, Jang HG, Hwang SB, Har DH, Moon YL, Chung MS. Two-dimensional sectioned images and three-dimensional surface models for learning the anatomy of the female pelvis. Anat Sci Educ. 2013; 6(5):316–323. PMID:
23463707.

10. Park JS, Chung MS, Shin DS, Har DH, Cho ZH, Kim YB, et al. Sectioned images of the cadaver head including the brain and correspondences with ultrahigh field 7.0 T MRIs. Proc IEEE Inst Electr Electron Eng. 2009; 97(12):1988–1996.

11. You Y, Kim CY, Kim SK, Chung BS, Har D, Choi J, et al. Advanced-sectioned images obtained by microsectioning of the entire male body. Clin Anat. 2022; 35(1):79–86. PMID:
34591338.

12. You Y, Park JS. A novel human brainstem map based on true-color sectioned images. J Korean Med Sci. 2023; 38(10):e76. PMID:
36918030.

13. Kim SK, Hur MS, Park JS. Real color sectioned images and correspondence with ultrasound images of the palmar wrist. Appl Sci. 2022; 12(1):299.

14. Park JS, Jung YW. Peeled images and sectioned images from real-color volume models of foot. Surg Radiol Anat. 2021; 43(1):37–43. PMID:
32676743.

15. Kim CY, Park JS, Chung BS. Real color model of a cadaver for deep brain stimulation of the subthalamic nucleus. Appl Sci. 2021; 11(11):4999.

16. Chung BS, Park HS, Park JS, Hwang SB, Chung MS. Sectioned and segmented images of the male whole body, female whole body, male head, and female pelvis from the Visible Korean. Anat Sci Int. 2021; 96(1):168–173. PMID:
32803722.

17. Park JS. 2D browsing software and 3D PDF of canine ear based on real color sectioned images. Int J Morphol. 2020; 38(1):147–152.

18. Kwon K, Park JS, Shin BS. Virtual anatomical and endoscopic exploration method of internal human body for training simulator. J Korean Med Sci. 2020; 35(12):e90. PMID:
32233159.

19. Park JS. Cross-Sectional Atlas of the Human Head: With 0.1-mm Pixel Size Color Images. Berlin, Germany: Springer;2018.
20. Park JS, You Y. Cross-Sectional Atlas of Human Brainstem: With 0.06-mm Pixel Size Color Images. Singapore: Springer Nature Singapore;2023.
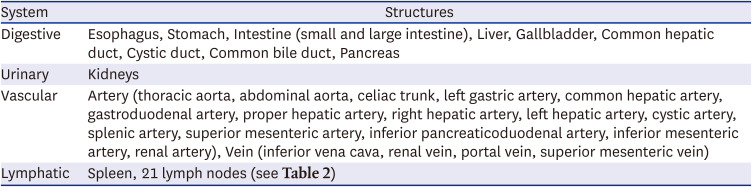
21. Harisinghani MG. Atlas of Lymph Node Anatomy. 2nd ed. Cham, Switzerland: Springer Nature Switzerland AG;2013. p. 59–88.
22. Chung BS, Park JS. Real-color volume models made from real-color sectioned images of Visible Korean. J Korean Med Sci. 2019; 34(10):e86. PMID:
30886552.

23. Moore KL, Dalley AF, Agur AM. Clinically Oriented Anatomy. 8th ed. Philadelphia, PA, USA: Wolters Kluwer;2018. p. 488–491.
24. Park JS, Chung MS, Hwang SB, Lee YS, Har DH. Technical report on semiautomatic segmentation using the Adobe Photoshop. J Digit Imaging. 2005; 18(4):333–343. PMID:
16003588.

25. FCAT. Terminologia Anatomica. Book & CD-ROM edition ed. New York, NY, USA: Thieme Medical Publishers;2002.
26. Ertürk MA, Raaijmakers AJ, Adriany G, Uğurbil K, Metzger GJ. A 16-channel combined loop-dipole transceiver array for 7 Tesla body MRI. Magn Reson Med. 2017; 77(2):884–894. PMID:
26887533.
27. Rahman N, Xu K, Budde MD, Brown A, Baron CA. A longitudinal microstructural MRI dataset in healthy C57Bl/6 mice at 9.4 Tesla. Sci Data. 2023; 10(1):94. PMID:
36788251.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download