INTRODUCTION
Hepatitis C virus (HCV) infection is a major cause of liver-related mortality and liver transplantation because of liver cirrhosis and liver cancer.
1 After the development of highly effective direct-acting antiviral (DAA) agents for HCV, the World Health Organization declared targets for global HCV elimination by 2030 of ≤ 5 new annual HCV infections/100,000 persons and ≤ 2 new annual HCV infections/100 persons who inject drugs (PWID), or an 80% reduction in the prevalence of HCV viremia from the 2015 baseline.
23 Because HCV is parenterally transmitted between people, PWID are at high risk of HCV infection by sharing injection syringes or injection/inhalation tools. Therefore, testing and treating HCV among PWID are top priorities for HCV elimination.
In South Korea, PWID are considered criminals because drug use is illegal, and only the number of drug offenders who are caught is reported, which almost doubled from 2010 to 2020.
4 Considering the far greater proportion of PWID who have not been charged with a drug offense, the increase in drug use is a threat to HCV elimination. However, there are no data on the epidemiology and treatment status of HCV infection among PWID, except for one study showing an HCV seroprevalence of 48.4% in 318 PWID during 2007–2010.
5
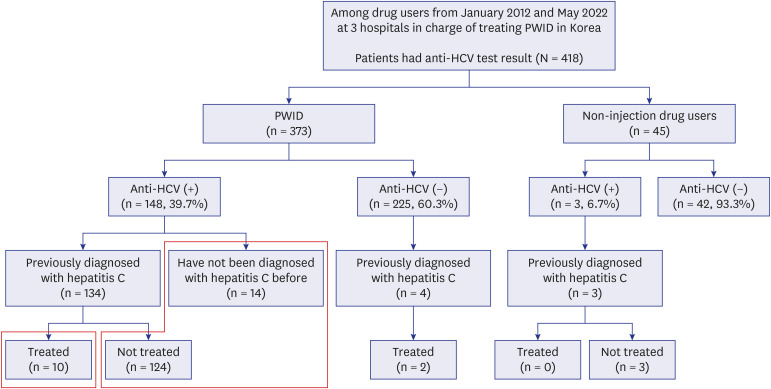
Currently, medical institutions that treat PWID are limited in South Korea, and approximately 90% of the care for diagnosed PWID is provided in only three hospitals: Bugok National Hospital, Incheon Chamsarang Hospital, and the National Forensic Hospital. Thus, this study aimed to retrospectively investigate HCV seroprevalence, factors related to HCV positivity, and treatment status among PWID between January 2012 and May 2022 in these three hospitals in South Korea.
DISCUSSION
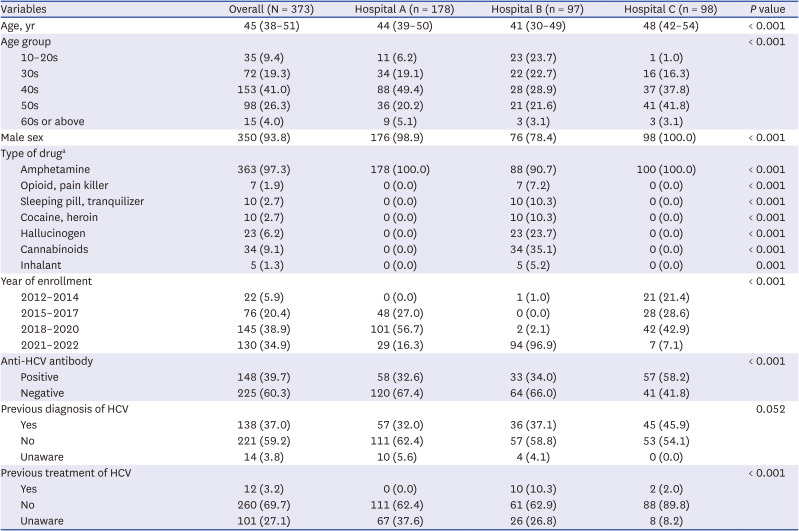
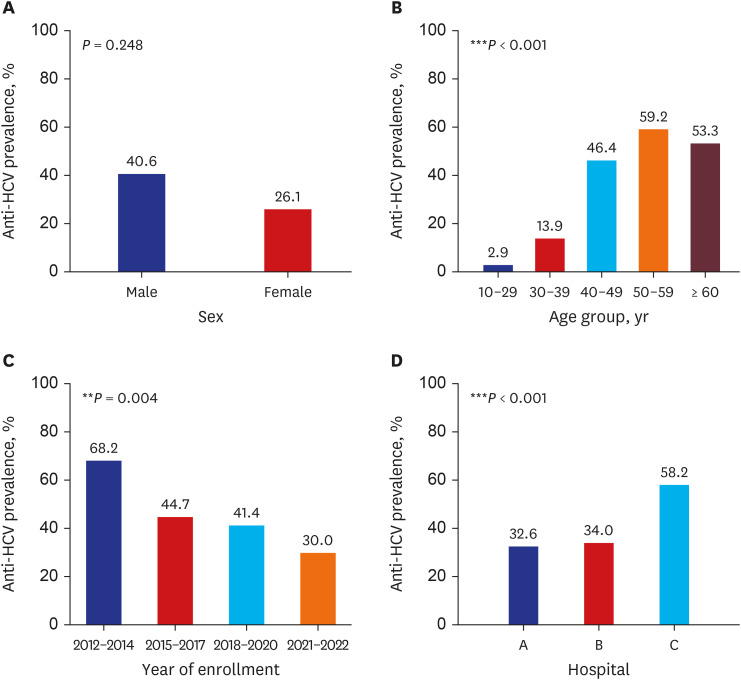
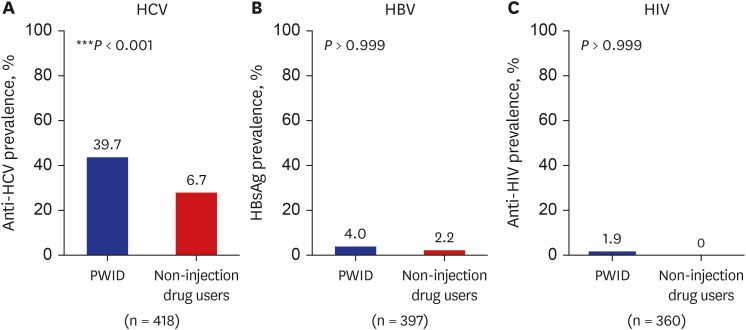
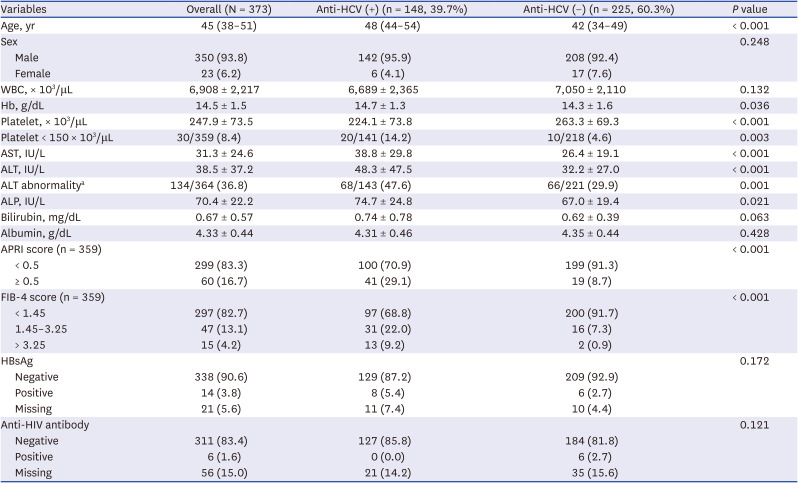
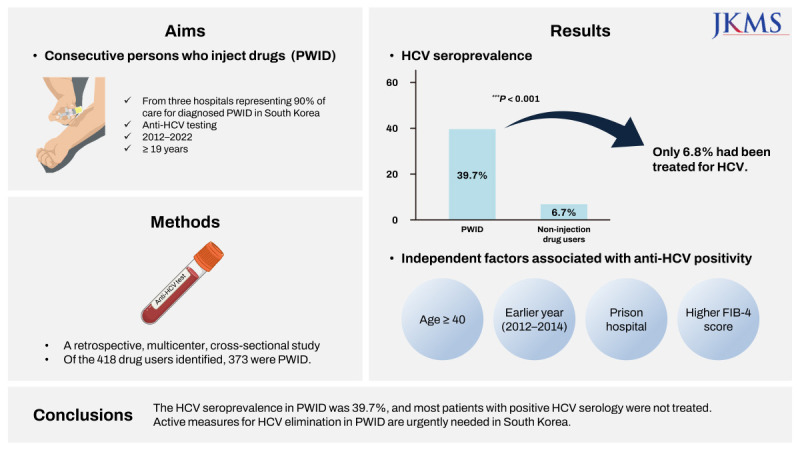
This study is the largest recent study of hepatitis C in PWID by enrolling PWID from three representative centers caring for 90% of known PWID in South Korea. The HCV seroprevalence was 39.7% in PWID, which was significantly higher than that in non-injection drug users (6.7%). The prevalence of HCV seropositivity increased with age, and decreased over time. Factors associated with HCV seropositivity were older age, earlier year of enrollment, attending the prison hospital, and a higher FIB-4 score. Additionally, 93.2% of HCV-seropositive PWID were not treated.
According to a modelling study that included 88 countries, the global prevalence of chronic HCV infection in PWID was 34.5% in 2017.
8 In terms of the population attributable fraction of the contribution of injection drug use to HCV transmission, if the increased risk of HCV transmission among PWID was removed, approximately 43% of incident HCV infections would be prevented from 2018 to 2030. In the modelling study, the population attributable fraction due to injection drug use was higher in high-income countries (79%) than in low- and middle-income countries (38%), where HCV transmission is driven by other factors such as unsterile medical injections or community risks, including tattooing and body piercings.
8 However, the study did not include South Korea because of the lack of data on PWID.
In this study, the HCV seroprevalence in Korean PWID was 39.7%, which is consistent with the global prevalence range, although the prevalence of HCV viremia in the PWID population is unknown. Considering that the HCV seroprevalence in the general population of South Korea was reported to be 0.6% in 2015–2019,
9 the HCV seroprevalence in PWID is 57 times higher than that in the general population, confirming that PWID is a high-risk population that should be targeted by HCV elimination programs. According to a previous study, the HCV seroprevalence of 318 PWID during 2007–2010 (89% male, mean age 42 years) was 48.4%, with a viremic proportion among the HCV-seropositive PWID of 98.1%.
5
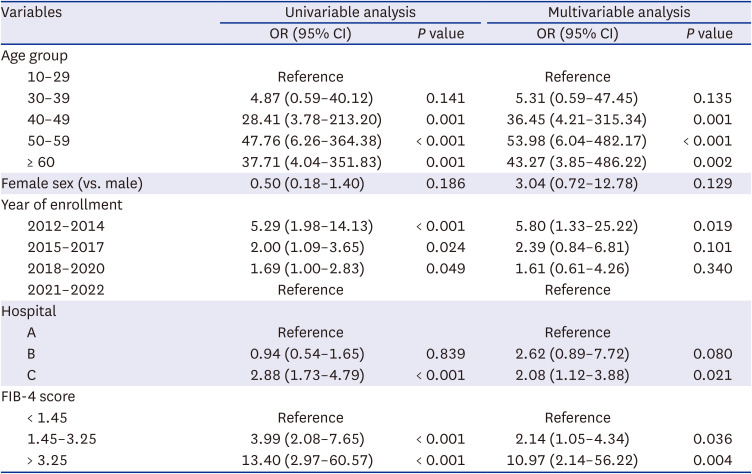
This study showed a gradual decrease in HCV seroprevalence according to the enrollment year. Improved education and awareness that HIV and HCV can be transmitted through syringe sharing during drug use may have contributed to the decline in anti-HCV seropositivity. In addition, as the recent intravenous (IV) drug purchase is made through social network service, it is expected that IV drug administration will be done by individuals or small groups more than before. Lastly, in 2021–2022, group IV drug overuse at nightlife facilities may have decreased due to social gathering restrictions during the coronavirus disease pandemic.
The HCV seroprevalence in PWID increased with age. This may be related to an increased number of injections with age. Moreover, HCV seroprevalence in the general population also increased with age (0.2–0.26%, 0.23–0.31%, 0.4–0.6%, 0.65–0.9%, and > 1% in the 20–29-years, 30–39-years, 40–49-years, 50–59 years, and ≥ 60-years age groups, respectively).
9101112 It is shown that the HCV seroprevalence was more than 100 times higher in young PWID than in the general population. This raises concern regarding the future HCV epidemiology in South Korea because the number of known Korean drug abusers increased from 9,732 in 2010 to 18,050 in 2020, and 50% of drug offenders were aged 20–39 years, with a rapid increase in the number aged < 20 years.
4
Among the three hospitals in this study, the HCV seroprevalence in the prison hospital was higher than that of the other two hospitals, and 21/22 of the patients enrolled from 2012 to 2014 were enrolled at the prison hospital. PWID have high rates of incarceration due to the illegality of drug use and high frequency of drug-related crimes, leading to a high HCV infection rate among prisoners.
1314 This finding suggests that prisons may be an effective target for HCV elimination in South Korea.
In the present study, 6.8% of PWID with positive anti-HCV results had a history of HCV treatment. Patients under the age of 40 years, recently registered, or from the private hospital, were more likely to have been treated. The higher rate of treatment in young people is promising but needs to be raised further considering their activity and transmission potential. Although PWID enrolled in 2020–2022 and those attending the private hospital B were also more likely to have been treated, there may be confounding because most of the PWID at the private hospital were enrolled in 2021 or 2022. Young (aged < 40 years), recently enrolled, private hospital patients have a high awareness of HCV and are highly motivated for HCV treatment. Since DAA with short treatment duration and high efficacy was recently introduced, it is necessary to establish a customized micro-elimination strategy considering the situation of PWID in Korea. Education and publicity about curable hepatitis C is needed for PWID and program managers. It is urgent to support treatment expenses for the economically vulnerable class among PWID.
In the present study, non-PWID drug users showed 6.8% of anti-HCV positivity which is higher than that in general population in South Korea (0.5–0.6%).
9 Similar to this study, previous studies showed 4.2–17.0% of anti-HCV positivity in non-PWID drug users, and the anti-HCV positivity decreased over time.
151617 Non-PWID drug users who consume illicit drugs often share paraphernalia used to snort and/or smoke the substance, and HCV RNA has been detected in pipes, nasal secretions, and snorting apparatuses.
16
A care cascade analysis of the 2015 health checkup population aged 20 years or older in South Korea (n = 268,422) showed that among 1,359 HCV-seropositive people, HCV RNA testing was performed in 60% and 10.9% were cured with interferon-based treatment.
10 In a prospective multicenter HCV cohort study conducted in South Korea, the treatment rate was 56.1% during 2015–2017 after DAA became available.
18 In 2019, the treatment rate of patients with newly diagnosed HCV viremia was 58.1%.
9 Moreover, recent studies have shown that the treatment uptake and sustained virologic response rate of DAA did not differ between PWID and non-PWID with chronic HCV infection.
1920 Therefore, the treatment of HCV infection in PWID should be scaled-up urgently in South Korea.
This study has several limitations. First, this study is a retrospective nature and it was not possible to collect data for the same period year by hospital. Therefore caution is needed to interpret anti-HCV positivity rates over time. However, positive rate of anti-HCV showed a trend of decreasing over time in hospital A and C, respectively. Second, because the data were collected from psychiatric hospitals, HCV RNA testing and abdominal ultrasound results were not available. Therefore, the prevalence of HCV viremia and the severity of liver disease could not be evaluated. Third, this was a cross-sectional study using data at the time patients were registered as PWID at each hospital and tested for HCV antibodies, and there was no follow-up information on whether they received HCV treatment later. Lastly, owing to the retrospective nature of this study, the known diagnosis and treatment rate of HCV estimated from chart review may not accurately reflect current values. However, this is the only available source of data for the evaluation of HCV seroprevalence in Korean PWID, covering the three hospitals responsible for approximately 90% of PWID care in South Korea.
In conclusion, the HCV seroprevalence in PWID was 39.7%, and most patients with positive HCV serology were not treated. Therefore, prospective studies of HCV epidemiology and active measures for HCV elimination in PWID are urgently needed in South Korea.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download