INTRODUCTION
Blood and gingival crevicular fluid must be controlled during restorative procedures, as contamination with blood and moisture adversely impacts hybrid layer formation, as well as the resulting bond strength between restorative materials and the tooth structure [
12]. Although rubber dam application can provide good control of the restoration area in certain clinical scenarios, this may not always be feasible, especially when dealing with class V cavities or subgingival margins [
3].
Another method of controlling blood and moisture is the use of topical hemostatic agents that act either as vasoconstrictors (such as epinephrine) or blood coagulation factors (such as aluminum potassium sulfate, ferric sulfate, and iron solutions) [
4]. Aluminum chloride at a concentration of 5% to 25% is commonly used as an astringent in solution or gel form [
4]. It acts by constricting damaged vessels, enabling the clinician to establish a dry field for bonding procedures. Several studies evaluating the effects of aluminum chloride-containing hemostatic agents on bond strength have indicated that contamination with hemostatic agents can significantly decrease bond strength [
56]. One reason for this decrease is the acidic nature of these substances (pH = 0.7 to 3), which can remove the smear layer and alter the dentin surface morphology [
7]. Contaminants such as residual aluminum can also obstruct the dentinal tubules and subsequently inhibit the inward flow of resin monomers [
8].
Universal or multi-mode adhesives have recently been introduced. These can be used in either a 2-step etch-and-rinse mode or a 1-step self-etch mode. This versatility gives the practitioner a choice of bonding techniques for use in clinical situations.
Few studies have been conducted to evaluate the bonding strengths of new universal adhesives to hemostatic agent-contaminated dentin. Thus, the purpose of this study was to determine the effect of aluminum chloride-containing hemostatic on the shear bond strength (SBS) of different adhesives, including universal adhesives to dentin. The null hypotheses tested were that 1) an aluminum chloride-containing hemostatic agent would have no effect on the dentin SBS values of various adhesive systems, 2) the application mode of All-Bond Universal adhesive would have no effect on the dentin SBS to hemostatic agent-contaminated dentin, and 3) the SBS measurements of various adhesive systems to hemostatic agent-contaminated dentin would not be affected by thermocycling.
MATERIALS AND METHODS
Sample preparation
This study was approved by the Institutional Review Board of Wonkwang Dental Hospital, Wonkwang University, Iksan, Korea (WKIRB-201512-BR-006). In this study, 80 extracted noncarious human molars were used. The teeth were stored in a 0.4% chloramine-T solution and used within 3 months of extraction. The teeth were prepared in sections perpendicular to the long axis of the crown to a level 2 mm below the dentinoenamel junction using a low-speed diamond saw (IsoMet; Buehler, Lake Bluff, IL, USA). The prepared teeth were embedded in cylindrical molds using a self-curing acrylic resin (Ortho-Jet; Lang Dental Manufacturing Co. Inc., Wheeling, IL, USA), allowing the occlusal surfaces of the dentin to be exposed. The exposed surfaces were ground with 600-grit abrasive paper (CC261; DEERFOS, Seoul, Korea) for 30 seconds to create the smear layer. The occlusal surfaces were subsequently divided into 2 parts mesiodistally, and the bonding procedures were performed on each part.
The prepared specimens were randomly divided into 2 main groups according to the application of the hemostatic Traxodent Hemodent paste displacement system (Premier Dental Products Co., Plymouth Meeting, PA, USA) prior to the application of dentin adhesive.
Control group (C): No contamination.
Hemostatic agent contamination (H): Traxodent hemostatic agent paste was applied to the dentin surfaces and pressed lightly with a cotton pellet to ensure close contact with those surfaces according to the manufacturer’s instructions. After 2 minutes, the hemostatic agent was rinsed thoroughly for 30 seconds and air-dried for 10 seconds.
Each group was divided into 4 subgroups based on the adhesive system used, as shown in
Figure 1. The materials used in this study are presented in
Table 1, and the tooth treatment procedure by subgroup, detailed below, is briefly described in
Table 2.
Figure 1
Schematic representation of the experimental procedure and testing groups.
SBER, Scotchbond Multi-Purpose; CLSE, Clearfil SE Bond; ALER, All-Bond Universal in etch-and-rinse mode; ALSE, All-Bond Universal in self-etch mode; SBS, shear bond strength; SEM, scanning electron microscopy.
Table 1
Composition and manufacturers of materials used in this study
|
Materials |
Manufacturer |
Lot number |
Composition |
|
Traxodent Hemodent Paste Retraction System |
Premier Dental Products Co., Plymouth Meeting, PA, USA |
71156 |
15% aluminum chloride, montmorillonite, fumed silica, potassium nitrate |
|
Ultra-Etch |
Ultradent, South Jordan, UT, USA |
ET523137 |
35% phosphoric acid, cobalt aluminate blue spinel, cobalt zinc aluminate blue spinel |
|
Scotchbond Multi-Purpose |
3M ESPE, St. Paul, MN, USA |
Primer: N591853 |
Primer: HEMA, polyalkenoic acid, copolymer (Vitrebond) |
|
Bond: N557534 |
Adhesive: Bis-GMA, HEMA, dimethacrylates, initiators |
|
Clearfil SE Bond |
Kuraray, Osaka, Japan |
Primer: 01247A |
Primer: 10-MDP, HEMA, hydrophilic dimethacrylate, water |
|
Bond: 01877B |
Bond: 10-MDP, Bis-GMA, HEMA silanated colloidal silica |
|
All-Bond Universal |
Bisco, Schaumburg, IL, USA |
1400004366 |
10-MDP phosphate monomer, HEMA, bis-GMA, ethanol, water, initiators |
|
Filtek Z350XT (shade: A1) |
3M ESPE, St. Paul, MN, USA |
7018A1E |
Bis-GMA, UDMA, TEGDMA, Bis-EMA, PEGDMA, silica filler, zirconia filler, zirconia/silica (aggregated) |
Table 2
Dentin surface treatment and application procedures
|
Group |
Application procedure |
|
SBER |
1. Dentin surfaces were acid-etched with Ultra Etch (Ultradent, South Jordan, UT, USA), 35% phosphoric acid for 15 seconds, rinsed with water for 15 seconds, and subsequently blotted dry. |
|
2. Scotchbond Multipurpose (3M ESPE, St. Paul, MN, USA) primer was applied to the dentin and gently air-dried for 5 seconds. |
|
3. Adhesive was applied, gently air-dried, and light-cured for 10 seconds. |
|
CLSE |
1. Clearfil SE Bond (Kuraray, Osaka, Japan) primer was applied to dentin, left for 20 seconds, and gently air-dried. |
|
2. Adhesive was applied for 15 seconds and then cured for 10 seconds. |
|
ALER |
1. Dentin surfaces were etched with Ultra Etch for 15 seconds, rinsed with water for 15 seconds, and blotted dry. |
|
2. All-Bond Universal (Bisco, Schaumburg, IL, USA) was applied to the dentin in 2 separated coats, the dentin surfaces were scrubbed for 10–15 seconds per coat, and excess solvent was evaporated by thoroughly air-drying for at least 10 seconds. |
|
3. The adhesive was light-cured for 10 seconds. |
|
ALSE |
1. All-Bond Universal was applied identically as in group ALER without etching. |
Scotchbond Multi-Purpose (SBER): Dentin surfaces were acid-etched with 35% phosphoric acid (Ultra-Etch; Ultradent, South Jordan, UT, USA) for 15 seconds, rinsed with water for 15 seconds, and subsequently blotted dry. Scotchbond Multi-Purpose (3M ESPE, St. Paul, MN, USA) primer was applied to the dentin and gently air-dried for 5 seconds. Adhesive was then applied, gently air-dried, and light-cured for 10 seconds.
Clearfil SE Bond (CLSE): Clearfil SE Bond (Kuraray, Osaka, Japan) primer was applied to the dentin, left for 20 seconds, and gently air-dried. Adhesive was applied for 15 seconds and then cured for 10 seconds.
All-Bond Universal etch-and-rinse mode (ALER): Dentin surfaces were etched with Ultra-Etch for 15 seconds, rinsed with water for 15 seconds, and blotted dry. All-Bond Universal adhesive (Bisco, Schaumburg, IL, USA) was applied to the dentin in 2 separate coats, the dentin surfaces were scrubbed for 10 to 15 seconds per coat, and excess solvent was evaporated via thorough air-drying for at least 10 seconds. The adhesive was then light-cured for 10 seconds.
All-Bond Universal self-etch mode (ALSE): All-Bond Universal was applied identically as in the ALER group, but without etching.
Adhesives were light-cured with a light-emitting diode (LED) light-curing unit (Elipar S10; 3M ESPE). The light intensity was 1,000 ± 40 mW/cm2. The cylinders of the resin composite (Filtek Z350 XT; 3M ESPE) in a cylindrical Teflon mold (2.38 mm in diameter × 3 mm in height) were placed over the bonding surface of each tooth according to ISO 29022:2013. Specimens were stored in distilled water for 24 hours at room temperature. Half of the specimens were thermocycled in a water bath (Rika-Kogyo, Hachioji, Japan) for 30,000 cycles between 5°C and 55°C with a 30-second dwell time and a 30-second transfer time to simulate the conditions of the oral cavity (groups CT and HT).
Shear bond strength test and statistical analysis
The SBS was measured using a shear bond tester (T-63010K; Bisco) with a half-round notch at a crosshead speed of 1 mm/min until failure. The SBS values were recorded in MPa. The SBS was calculated by dividing the maximum load at failure by the cross-sectional surface area. The SBS test data were normally distributed and were statistically analyzed using 1-way analysis of variance to compare the effects by bonding system. Additionally, the data were analyzed using the Student’s t-test to determine the effects of hemostatic agent and thermocycling. The Tukey post hoc test was used. The sample size for the in vitro study was determined using G*Power 3.1 software (University of Düsseldorf, Düsseldorf, Germany). A power analysis using the F test was applied (effect size = 0.5, power = 0.95) to identify the required sample sizes. The Shapiro–Wilk test was used to assess the normality of distribution prior to the statistical analysis. Statistical significance was considered to be indicated by a p value of less than 0.05. All statistical analyses were performed using SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
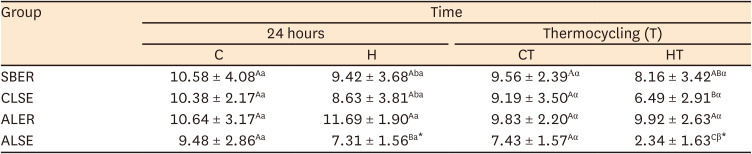
The results of the SBS test are shown in
Table 3. At 24 hours, no statistically significant differences in SBS were present between the C and H groups for all adhesive systems tested. After thermocycling, a statistically significant difference was noted between the CT+ALSE and HT+ALSE groups (
p < 0.05). No statistically significant differences were found within group C; however, within group H, the SBS of the H+ALSE specimens was significantly lower than that of the H+ALER group (
p < 0.05). Within the CT subgroups, no statistically significant differences were present among adhesives, and within the HT subgroups, no significant differences in SBS were found between HT+SBER and HT+ALER. Notably, the SBS value of HT+ALSE was the lowest among the adhesive systems (
p < 0.05). When comparing the specimens before and after thermocycling, the SBS of HT+ALSE was significantly lower than that of H+ALSE (
p < 0.05). The subgroups of SBER exhibited no significant differences in SBS regardless of treatment and thermocycling. The SBS of HT+CLSE was significantly lower than that of H+CLSE (
p < 0.05).
Table 3
Mean shear bond strength values ± standard deviation (MPa) (n = 10)
|
Group |
Time |
|
24 hours |
Thermocycling (T) |
|
C |
H |
CT |
HT |
|
SBER |
10.58 ± 4.08Aa
|
9.42 ± 3.68Aba
|
9.56 ± 2.39Αα
|
8.16 ± 3.42ABα
|
|
CLSE |
10.38 ± 2.17Aa
|
8.63 ± 3.81Aba
|
9.19 ± 3.50Aα
|
6.49 ± 2.91Bα
|
|
ALER |
10.64 ± 3.17Aa
|
11.69 ± 1.90Aa
|
9.83 ± 2.20Aα
|
9.92 ± 2.63Aα
|
|
ALSE |
9.48 ± 2.86Aa
|
7.31 ± 1.56Ba*
|
7.43 ± 1.57Aα
|
2.34 ± 1.63Cβ*
|
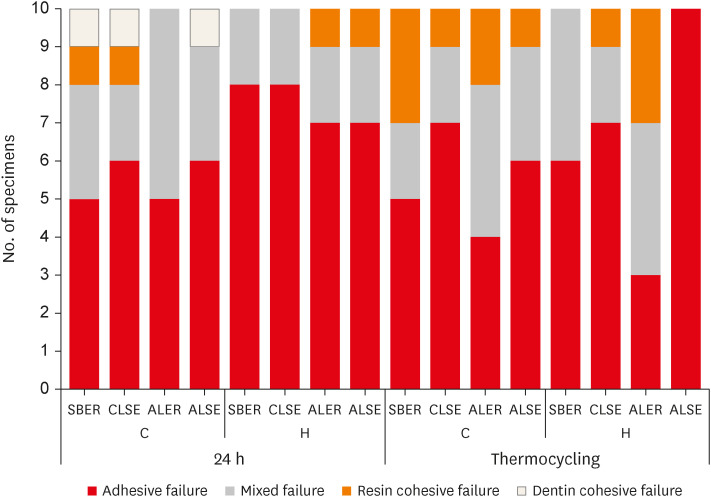
The failure modes are shown in
Figure 2. A higher percentage of adhesive failure was observed after hemostatic agent application in all groups except HT+ALER. All HT+ALSE specimens displayed adhesive failure.
Figure 2
Failure modes after the shear bond strength test. The most prominent failure mode was adhesive failure except for HT+ALER. All HT+ALSE specimens showed adhesive failure.
C, control; H, hemostatic agent application; T, thermocycling; SBER, Scotchbond Multi-Purpose; CLSE, Clearfil SE Bond; ALER, All-Bond Universal in etch-and-rinse mode; ALSE, All-Bond Universal in self-etch mode.
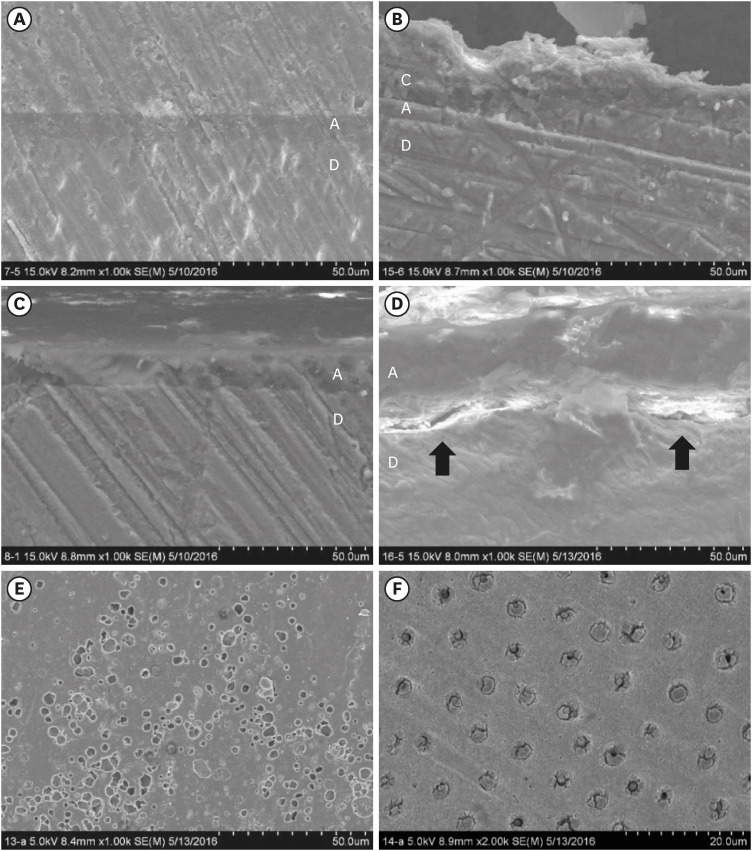
For the specimens for which All-Bond Universal adhesive was used, the morphologic characteristics of the dentin/adhesive interfaces are shown in scanning electron microscopy (SEM) (S-4800; Hitachi, Tokyo, Japan) micrographs (
Figure 3). No differences were seen in the dentin/adhesive interfaces of the H+ALER and HT+ALER groups (
Figure 3A and B). However, gaps between the dentin and the ALSE adhesive layer were observed after thermocycling (
Figure 3D). In the SEM image of HT+ALSE, numerous pores were noted above the adhesive layers (
Figure 3E).
Figure 3
Scanning electron microscopy images of specimens with All-Bond Universal after Traxodent application (A-E; ×1,000, F; ×2,000). (A) Image of H+ALER specimen. (B) Image of HT+ALER specimen. (C) Image of H+ALSE specimen. (D) Image of HT+ALSE specimen. Gaps were observed between the dentin and adhesive layer (black arrow). (E) Adhesive layers of HT+ALSE specimen. Multiple pores were noted above the adhesive layers of the HT+ALSE tooth. (F) Dentin surfaces of HT+ALSE specimen in the area where adhesive failure occurred. Obstructed dentinal tubules were shown on the dentin surface of the HT+ALSE tooth.
H, hemostatic agent application; T, thermocycling; ALER, All-Bond Universal in etch-and-rinse mode; ALSE, All-Bond Universal in self-etch mode.
DISCUSSION
Hemostatic agents are acidic (pH = 0.7 to 3.0) [
9], with hydrophilic characteristics that can contaminate every stage of the bonding procedure and subsequently cause morphologic changes in dentin surfaces [
710]. In addition, contaminants, such as residual particles of the hemostatic agents, can obstruct the flow of resin monomers into the dentinal tubules. Thus, hemostatic agents can negatively affect bond strength [
11].
In the present study, the first and second null hypotheses were rejected. At 24 hours, hemostatic agents reduced the SBS of the H+ALSE specimens significantly more than the H+ALER specimens. This result aligned with the findings of a previous study by Kuphasuk
et al. [
6], who reported a decrease in the SBS of a self-etching adhesive after the application of an aluminum chloride hemostatic agent.
The hemostatic agent, the Traxodent Hemodent Paste Retraction System (Premier Dental Products Co.), was selected for the present study based on its specific formula. The Traxodent system consists of an aluminum chloride topical paste and cotton caps used to efficiently compress the paste material for retraction of the gingival sulcus. Traxodent paste is a clay-based paste that contains 15% aluminum chloride with 85% fillers in addition to water and various modifiers [
12]. The fillers are composed of montmorillonite and fumed silica. Montmorillonite, from the smectite group of clay minerals, can absorb a large amount of water due to its layered structure [
13]. Upon exposure to the blood, it absorbs water and thus concentrates the remaining clotting factors [
14]. When montmorillonite is exposed to blood, it swells into a clay paste with high plasticity and strong adhesive properties [
15].
In the current study, Traxodent paste was lightly pressed with cotton pellets to simulate the use of a cotton cap with paste and was applied for 2 minutes in accordance with the manufacturer’s recommendations. As the manufacturer did not stipulate rinsing time, the paste was rinsed off with a water spray for 30 seconds as performed in previous studies [
61116].
Previous studies commonly involved a solution or gel containing 25% aluminum chloride; however, in the present study, a paste with 15% aluminum chloride was used [
5611161718]. The clinical hemostatic performance of 15% aluminum chloride paste is known to resemble that of the 25% solution; however, few studies have been published evaluating the dentin bonding strength to 15% aluminum chloride paste-contaminated dentin [
19]. The results of the present study seem to corroborate the results obtained using the 25% aluminum chloride solution. This finding suggests that residual aluminum contaminants are similar between the 15% aluminum chloride paste and the 25% aluminum chloride solution/gel, as evidenced by the paste’s strong adhesive properties. Furthermore, rinsing for 30 seconds may be insufficient to remove the paste preparation, unlike the solution or gel materials.
In a study by Ayo-Yusuf
et al. [
20], hemostatic agents appeared to remove the smear layer and formed a granular precipitate on the dentin surface. The researchers concluded that the exposure of the dentin surface to hemostatic agents altered its morphology and reduced its susceptibility to etching [
20]. As previously reported, an aluminum chloride-contaminated tooth can inhibit hydroxyapatite demineralization via the exchange of calcium for aluminum in hydroxyapatite, resulting in the formation of Al(OH)
2H
2PO
4, which is insoluble and has an increased resistance to acid [
21]. Because of the weak acidity of the self-etch primers relative to phosphoric acid, they would reasonably be expected not to sufficiently remove aluminum contaminants, and their penetration to deeper areas of the dentin tubules would be impossible. In etch-and-rinse mode, phosphoric acid can demineralize the dentin and remove the remaining contaminants from the dentin surface. Phosphoric acid in etch-and-rinse mode may therefore function as a cleaning agent for aluminum chloride-contaminated dentin [
22].
The third null hypothesis, that the SBS values of adhesive systems to hemostatic agent-contaminated dentin are unaffected by thermocycling, was rejected.
Thermocycling is a common aging method used to investigate bond stability. The use of 10,000 cycles corresponds to approximately 1 year of clinical functioning [
23]; thus, the 30,000 cycles used in this study simulate approximately 3 years of clinical function.
After thermocycling, the HT+SBER, HT+ALER and HT+CLSE groups exhibited bond durability; however, a significant decrease in the SBS of HT+ALSE was observed. Comparing adhesive systems, the SBS values associated with etch-and-rinse modes (HT+SBER and HT+ALER) were higher than those of self-etch modes (HT+CLSE and HT+ALSE). The SBS of HT+ALSE was the lowest among the adhesive systems, constituting a significant finding.
Depending on the pH, Clearfil SE Bond can be classified as a mild self-etch adhesive system (as it is at a pH of 2.0), whereas All-Bond Universal is classified as ultra-mild (at a pH of 3.1). Etching aggressiveness is related to acidity, and milder self-etch adhesives therefore show a lower capacity to penetrate thick smear layers than do stronger ones. In conclusion, Clearfil SE Bond showed superior performance to All-Bond Universal in self-etch mode after hemostatic agent application due to the relatively hydrophobic properties and lower pH values of Clearfil SE Bond.
Clearfil SE Bond is a mild 2-step self-etching adhesive system, while All-Bond Universal is classified as an ultra-mild multi-mode adhesive. These 2 materials contain a 10-methacryloyloxydecyl dihydrogen phosphate (MDP) monomer, and 10-MDP is known to chemically interact with hydroxyapatite to form a hydrolytically stable bond with calcium [
24]. However, the present study demonstrated reduced bond durability to hemostatic-contaminated dentin for the self-etch mode adhesives, especially ALSE, perhaps because they contain hydroxyethyl methacrylate (HEMA) as well as 10-MDP. Tay
et al. [
25] demonstrated that when water was incompletely evaporated from dentin, porous hydrogels were formed through copolymerization with HEMA and acidic monomers. In addition, the presence of water may cause insufficient polymerization in the resin matrix [
25]. These insufficiently polymerized regions may allow water permeation, affecting the bonding durability. HEMA may also compete with 10-MDP by binding to the calcium on the surface of apatite crystallites, leading to reduced nano-layering of 10-MDP-calcium salts in the resin–dentin interface [
26].
In the SEM images, no differences were observed in the dentin/adhesive interfaces of the etch-and-rinse mode application group before and after thermocycling (H+ALER vs. HT+ALER). Gaps between hemostatic-contaminated dentin and adhesive were observed in the self-etch group after thermocycling (HT+ALSE). Additionally, these gaps manifested as pores above the adhesive layers of the HT+ALSE specimens and may form due to the permeability of the adhesive to hemostatic-contaminated dentin.
The low bond strengths of the HT+ALSE specimens were also indicated by the failure mode. All specimens exhibited adhesive failures at the dentin interface, which may indicate weak resin-dentin bonding. After thermocycling, cohesive failure in composite resin increased in all groups except HT+ALSE. The presence of water may cause swelling and lead to a mechanical decrease in the resin integrity [
27].
Clinically, hemostatic agents are frequently used for bleeding control before composite resin restoration treatment. Given the wide use of so-called universal adhesives, whether hemostatic agents affect their adhesion is a clinically important issue. This study revealed that when hemostatic agents are used and All-Bond Universal is applied, it is advantageous to incorporate a separate etching step. A limitation of this study is that the hemostatic agent was used without blood contamination. If blood and hemostatic agents were applied sequentially, the dentin SBS could be lower than observed here because blood and hemostatic agents become coagulated and resistant to washout.










 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download