Abstract
Objective
Chronic subdural hematoma (CSDH) patients using antithrombotic agents (AT) at high risk for cardiovascular disease are increasing. The authors aimed to analyze the factors influencing outcome by targeting patients using AT and to establish a desirable treatment strategy.
Methods
A retrospective analysis was performed on data from 462 patients who underwent burr hole trephination (BHT) surgery for CSDH at five hospitals from March 2010 to June 2021. Outcomes included incidence of postoperative acute bleeding, recurrence rate, and morbidity or mortality rate. Patients were divided into the following four groups based on their history of AT use : no AT. Only antiplatelet agents (AP), only anticoagulants (AC), both of AP and AC. In addition, a concurrent literature review was conducted alongside our cohort study.
Results
Of 462 patients, 119 (119/462, 25.76%) were using AT. AP prescription did not significantly delay surgery (p=0.318), but AC prescription led to a significant increase in the time interval from admission to operation (p=0.048). After BHT, AP or AC intake significantly increased the period required for an in-dwelling drain (p=0.026 and p=0.037). The use of AC was significantly related to acute bleeding (p=0.044), while the use of AP was not (p=0.808). Use of AP or AC had no significant effect on CSDH recurrence (p=0.517 and p=1.000) or reoperation (p=0.924 and p=1.000). Morbidity was not statistically correlated with use of either AP or AC (p=0.795 and p=0.557, respectively), and there was no significant correlation with mortality for use of these medications (p=0.470 and p=1.000).
Chronic subdural hematoma (CSDH) occurs frequently in the elderly and is thought to be related to trauma and to use of drugs such as antiplatelet agents (AP) or anticoagulants (AC) [8,20]. However, CSDH is a complex disease with diverse pathophysiology, not just traumatic causes. As the prevalence of cardiovascular disease (CVD) increases with global aging, the number of CSDH patients using antithrombotic agents (AT) is gradually increasing. Discontinuation of AT when CSDH is diagnosed is generally recommended. This aims to prevent progression of CSDH or to reduce the bleeding tendency during surgery, even though burr hole trephination (BHT) is a simple procedure with low bleeding risk, unlike other cranial surgeries. However, there is no definite guideline for perioperative use of AT [11,26]. Through this retrospective cohort analysis and review of literature, we suggest a guideline for treating CSDH in patients using AT.
The Catholic Medical Center Central Institutional Review Board approved the current study (approval No. XC22RIDI0012V).
From March 2010 to June 2021, a retrospective analysis was performed on data from 462 patients who underwent BHT for CSDH at five hospitals. The patient’s age, sex, hospitalization date, and surgery date were assessed; and the histories of hypertension, diabetes, and hyperlipidemia were evaluated. The medication history of antiplatelet agent and anticoagulant use, as well as the reason for medication, was determined. The brain computed tomography (CT) performed on postoperative day 1 was assessed for signs of acute hemorrhage, and the outpatient department monitored for recurrence for 6 months after the operation. Recurrence was defined as a case with increased subdural hematoma volume on CT within 6 months after surgery. Reoperation or conservative treatment was determined in consideration of patient’s neurological condition and various other circumstances. Since reoperation was not performed in all cases of recurrence, the recurrence rate and the reoperation rate were analyzed separately.
Patients were divided into the following four groups : those not taking AT, those taking only AP, those taking only AC, those taking both AP and AC.
Timing of surgery was determined by calculating the duration from admission to the operation after discontinuation of medication. Postoperative acute hemorrhage was defined as the presence of noticeable acute hemorrhage on brain CT performed within 24 hours after surgery. The duration of indwelling drainage catheter was calculated as the interval between the day of the surgery and the day the indwelling drainage catheter was removed.
To investigate the impact of preoperative AT discontinuation on recurrence and postoperative acute bleeding, patients were divided based on a discontinuation period of 1 to 4 days. Since the effect of AP medication lasts for 5 days, patients who discontinued AP for more than 5 days were excluded from further analysis.
Data acquired included the median patient age, the length of time between discontinuation of AT and surgery, and the length the postoperative period requiring drain maintenance. First and third quartile were confirmed using interquartile range. The chi square test was utilized for assessment of a correlation between hematoma density and pattern on the initial brain CT and for the presence of acute hemorrhage on postoperative CT with antiplatelet agent usages. The correlations between initial hematoma density and pattern and postoperative acute hemorrhage with anticoagulant usage were assessed by Fisher’s exact test. The correlations between recurrence and reoperation and medication history of AT were confirmed by chi-square and Fisher exact tests. Generalized logistic regression was used for analyzing the period from hospitalization to surgery and the length of drain usage. R studio (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria) was used.
The median age of the patients was 75 years (67.00–81.00 years), and 311 males (67.32%) and 151 females (32.68%) were included in the study. Hypertension was present in 262 patients (56.71%), 140 patients (30.30%) had diabetes, and 84 patients (18.18%) had hyperlipidemia. Liver disease affected 28 patients (6.06%), and 24 patients had renal disease (5.19%). A history of ischemic cerebral infarction was confirmed in 37 patients (8.01%), hemorrhagic stroke in three patients (0.06%), and both ischemic and hemorrhagic stroke in one patient (0.22%). A history of heart disease was present in 50 patients (10.82%), cancer in 31 patients (6.71%), and blood disease in 10 patients (2.16%). For CSDH, right side operation was performed in 183 cases, left side operation was performed in 201 cases, and bilateral operation was performed in 78 cases (Table 1).
Regarding medication history, 100 patients were using AP, 50 for secondary prevention of heart disease, and 37 for secondary prevention of ischemic stroke. The other patients took AP for primary prevention of stroke depending on medical histories of issues such as hypertension or diabetes mellitus. Seventeen patients were using AC, and four for secondary prevention of ischemic stroke and the others for secondary prevention of heart disease. Two patients were using both an AP and an AC together (AP+AC) because of history of both heart disease and ischemic stroke. Details regarding the types of AT administration are outlined in Table 2.
Patients with no medication history of AT required a median of 1 day (0.00–2.00) of hospitalization before surgery, and the same was true for patients taking AP (0.00–3.00). A median of 2 days (1.00–6.00) of presurgical hospitalization, for patients taking AC, was the longest interval of pre-surgical hospitalization. Of the two patients using AP+AC, one underwent surgery after 2 days and the other after 12 days (Fig. 1). Use of AP did not significantly delay BHT (p=0.318), but use of AC did significantly delay the operation (p=0.048). In patients taking AC, further analysis was performed depending on type. Being prescribed warfarin was of borderline significance (p=0.076) for a longer surgical delay, but there was no clear correlation between factor Xa inhibitor or thrombin inhibitor prescription (p=0.340 and p=0.558) and hospital stay prior to surgery.
The duration of postoperative subdural drainage via indwelling catheter was a median of 2 days (1.00–3.00) in patients with no medication history of AT, a median of 2 days (1.00–3.00) in the AP group, and a median of 3 days (2.00–3.00) in the AC group. In the association analysis, even though there was no difference in median, AP intake significantly increased the drain period (p=0.026), as did AC intake (p=0.037) (Fig. 2).
On brain CT performed the first day after surgery, acute hemorrhage was confirmed in 21.70% of the drug-free group, 19.00% of the AP group, 35.29% of the AC group, and all patients taking both AP+AC. AP intake had no effect (p=0.808), but AC intake was significantly associated with acute hemorrhage (p=0.044) (Table 3). We performed a subgroup analysis of patients taking AP. The period of AP discontinuation prior to surgery was not statistically significant with the incidence of acute hemorrhage on postoperative (p=0.161).
Recurrence occurred in 17.78% of cases of the no medication group, and reoperation was performed in 14.29%. Reoperation was performed in all cases of recurrence in the AP and AC groups, and the rates were 12.00% and 5.88%, respectively. Recurrence occurred in both patients taking combined AP+AC, and reoperation was performed in one of them. Regarding the association with recurrence, intake history of AP or AC had no significant effect (p=0.517 and p=1.000, respectively), and there was no effect on reoperation rate (p=0.924 and p=1.000) (Table 3). Additionally, a statistically significant positive correlation was observed between the preoperative AP discontinuation period and the recurrence rate (p=0.033; odds ratio, 1.658; 95% confidential interval, 1.036–2.673).
Morbidity occurred in 4.37% of patients with no medication history and included cerebral contusion, acute subdural hemorrhage, pneumonia, and abscess formation after BHT. Morbidity occurred in 5.00% of patients taking AP and included epidural hemorrhage, subarachnoid hemorrhage, and acute kidney injury after the operation. Morbidity occurred in one patient taking AC as acute subdural hemorrhage. There was also one case of morbidity as acute subdural hemorrhage in patients taking AP+AC. There was no statistical correlation between medication history and morbidity for AP or AC (p=0.763 and p=0.800, respectively). Mortality was confirmed in 2.04% of the no medication group, 3.00% of the AP group, and 0% of the AC and AP+AC groups. There was no significant correlation between mortality and intake of AP or AC (p=0.748 and p=0.992, respectively) (Table 3).
CSDH is a frequent disease in the population older than 70 years and is highly related to trauma, although it cannot be explained by a single mechanism. Regarding pathophysiology, there is a consensus that CSDH is not caused by acute bleeding directly but by complex interactions of various mechanisms such as association with inflammation, fluid collection due to osmotic change, and continuous bleeding [12,13,23]. BHT is the most popular surgical treatment option for CSDH [6,22]. Despite the complex pathophysiology of CSDH, BHT is a simple procedure requiring relatively minimal training. Although bleeding risk of the procedure is not high as other surgeries, most patients suspended their previously taken AT during the process of preparing for BHT. Based on this and the high frequency of CSDH in elderly populations with thromboembolic risk, this study was conducted to determine the best use of perioperative AT.
There are several retrospective studies that have analyzed the relationship between AT, especially AP, and the postoperative course of CSDH. Most of these studies concluded that AT use did not affect recurrence of CSDH after the operation [1,5,7,15,25]. One report showed a relationship between AP and recurrence of CSDH, but the recurrence rate in the article was relatively higher than in other articles, suggesting a bias [21]. Another study provided evidence that medication history of AC affected recurrence of CSDH; but, in the AT group, a larger preoperative hematoma volume was noted, with no effect of AP on recurrence [27]. In separate study, it was observed that while AT usage did not have a significant impact on recurrence in univariate analysis, an increase in odds ratio was identified in univariate regression analysis [5]. Like the results of our study (p=0.517, Table 3), the effect of AT usage on recurrence of CSDH is not clear (Table 4). Additionally, preoperative AP discontinuation period and recurrence rate showed a positive correlation in this study. This may reflect the paradoxical risk of discontinuation of AT previously revealed [2].
In existing literature, there are varying opinions on whether and when to stop antiplatelet medication before surgery for CSDH. In a retrospective study of patients taking AT, discontinuation of medication did not affect the clinical outcome [19]. Considering the characteristics of platelets, it is believed that the effectiveness of the medications would diminish when AP is discontinued for 5 days. However, a review article suggests that AP should be stopped 1 week before surgery, as with other major surgeries [17]. On the other hand, there are studies suggesting that even when discontinued for more than 3 days, it is possible to reduce the occurrence of postoperative acute hemorrhage [21,25]. In our study, there was no significant correlation between the duration of AT discontinuation before surgery and the occurrence of acute hemorrhage after surgery (Fig. 2).
A separate study found that early resumption group had a higher recurrence rate [15]. However, there are also important retrospective studies concerning thromboembolic complications after CSDH surgery in patients with history of AT. Most studies emphasize the importance of early resumption of AT after surgical intervention for CSDH [2,7,10,26]. Resumption timing of AT after operation did not affect recurrence of CSDH in two studies [7,26], and patients with history of AT use showed more frequent thromboembolic events after the operation in three studies [2,10,26] (Table 4). In our dataset, it was observed that AT was resumed in 25 cases, with a wide range of timeframes spanning from 2 days to 3 months. However, due to limited number of patients and the lack of detailed records regarding the exact timing of resumption, we were unable to perform an analysis on this aspect.
The importance of continuous use of AT is an emerging concept in our aging society. Regarding the risk of bleeding during surgery, studies on dosing and discontinuation guidelines for AP and AC for cardiac surgery and non-cardiac surgery are on-going; and guidelines are currently in place [18]. Major adverse cardiovascular event is a complication of surgery using general anesthesia and occurs in close to 5% of patients over the age of 45 years [25]. For patients aged 40 to 70 years at high risk for CVD, aspirin is also recommended for primary prevention [3]. In addition, lifelong AP prescription is recommended for secondary prevention in patients with CVD [14] . A more recent large observational study reported that maintenance of aspirin did not increase the risk of bleeding in noncardiac surgery [16]. Based on this, patients with CVD taking aspirin are recommended to maintain aspirin therapy alone when undergoing non-cardiac surgery [9,18]. The common belief is that maintaining medications, especially aspirin, is more helpful than discontinuation. Also, treating patients more actively rather than delaying surgery out of fear of recurrence of CVD and discontinuing medication is preferable.
In our study, patients who took AP or AC had longer postoperative maintenance of in-dwelling drains than those who did not take AT. Although more frequent acute bleeding was observed on postoperative CT in patients taking AC, there was no effect on recurrence or reoperation. Close observation and management of patients immediately after operation are essential for bleeding control. Although this study did not prove a clear difference between warfarin and other AC, direct oral anticoagulant (DOAC) therapy has a lower risk of bleeding than use of warfarin, a vitamin K antagonist; and DOAC is beneficial because there are fewer restrictions such as INR control [4]. The use of DOAC is increasing, and the risk of drug-induced bleeding in patients with CSDH is expected to decrease. Related studies are needed in the future.
In conducting a multi-center retrospective study, discontinuation of AT was performed at the discretion of individual surgeons. Also, there was the possibility of non-uniform data collection in the multicenter data collection process. Compared to AP, the analysis of AC was limited due to the small number of patients taking AC. Also, our dataset lacked information on resumption of AT and thromboembolic complications. To establish guidelines on the discontinuation and resumption of AT medications in CSDH, a larger prospective randomized study is necessary.
CSDH usually occurs in elderly patients under high risk of cardiovascular events. According to the results of this cohort analysis and literature review, maintenance of AT therapy might be more beneficial for CSDH patients with BHT considering its effect in minimizing the risk of thromboembolism. However, unlike AP, AC can increase the risk of acute bleeding after surgery. Thus, establishing a patient-specific strategy for discontinuation and resumption is necessary. If proper attention is given immediately after the operation through acute hemorrhage monitoring and establishment of postoperative hematoma drainage, there may be no significant difference in the patient’s prognosis, recurrence, and need for reoperation.
Notes
References
1. Abboud T, Dührsen L, Gibbert C, Westphal M, Martens T. Influence of antithrombotic agents on recurrence rate and clinical outcome in patients operated for chronic subdural hematoma. Neurocirugia (Astur : Engl Ed). 29:86–92. 2018.

2. Amano T, Takahara K, Maehara N, Shimogawa T, Mukae N, Sayama T, et al. Optimal perioperative management of antithrombotic agents in patients with chronic subdural hematoma. Clin Neurol Neurosurg. 151:43–50. 2016.

3. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 140:e596–e646. 2019.
4. Chan YH, See LC, Tu HT, Yeh YH, Chang SH, Wu LS, et al. Efficacy and safety of apixaban, dabigatran, rivaroxaban, and warfarin in asians with nonvalvular atrial fibrillation. J Am Heart Assoc. 7:e008150. 2018.

5. Choi J, Pyen J, Cho S, Kim J, Koo Y, Whang K. Influence of antithrombotic medication on the risk of chronic subdural hematoma recurrence after burr-hole surgery. J Korean Neurosurg Soc. 63:513–518. 2020.

6. Ducruet AF, Grobelny BT, Zacharia BE, Hickman ZL, DeRosa PL, Andersen KN, et al. The surgical management of chronic subdural hematoma. Neurosurg Rev. 35:155–169. discussion 169. 2012.

7. Fornebo I, Sjåvik K, Alibeck M, Kristiansson H, Ståhl F, Förander P, et al. Role of antithrombotic therapy in the risk of hematoma recurrence and thromboembolism after chronic subdural hematoma evacuation: a population-based consecutive cohort study. Acta Neurochir (Wien). 159:2045–2052. 2017.

8. Gaist D, García Rodríguez LA, Hellfritzsch M, Poulsen FR, Halle B, Hallas J, et al. Association of antithrombotic drug use with subdural hematoma risk. JAMA. 317:836–846. 2017.

9. Gerstein NS, Albrechtsen CL, Mercado N, Cigarroa JE, Schulman PM. A comprehensive update on aspirin management during noncardiac surgery. Anesth Analg. 131:1111–1123. 2020.

10. Guha D, Coyne S, Macdonald RL. Timing of the resumption of antithrombotic agents following surgical evacuation of chronic subdural hematomas: a retrospective cohort study. J Neurosurg. 124:750–759. 2016.

11. Hamou HA, Clusmann H, Schulz JB, Wiesmann M, Altiok E, Höllig A. Chronic subdural hematoma. Dtsch Arztebl Int. 119:208–213. 2022.
12. Holl DC, Volovici V, Dirven CMF, Peul WC, van Kooten F, Jellema K, et al. Pathophysiology and nonsurgical treatment of chronic subdural hematoma: from past to present to future. World Neurosurg. 116:402–411.e2. 2018.

13. Kawakami Y, Chikama M, Tamiya T, Shimamura Y. Coagulation and fibrinolysis in chronic subdural hematoma. Neurosurgery. 25:25–29. 1989.

14. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 45:2160–2236. 2014.

15. Kerttula S, Huttunen J, Leinonen V, Kämäräinen OP, Danner N. The effect of antithrombotic therapy on the recurrence and outcome of chronic subdural hematoma after burr-hole craniostomy in a population-based cohort. Acta Neurochir (Wien). 164:2699–2708. 2022.

16. Lewis SR, Pritchard MW, Schofield-Robinson OJ, Alderson P, Smith AF. Continuation versus discontinuation of antiplatelet therapy for bleeding and ischaemic events in adults undergoing non-cardiac surgery. Cochrane Database Syst Rev. 7:CD012584. 2018.

17. Mehta V, Harward SC, Sankey EW, Nayar G, Codd PJ. Evidence based diagnosis and management of chronic subdural hematoma: a review of the literature. J Clin Neurosci. 50:7–15. 2018.

18. Moster M, Bolliger D. Perioperative guidelines on antiplatelet and anticoagulant agents: 2022 update. Curr Anesthesiol Rep. 12:286–296. 2022.

19. Scerrati A, Germanò A, Trevisi G, Visani J, Lofrese G, D’Angelo L, et al. Timing of low-dose aspirin discontinuation and the influence on clinical outcome of patients undergoing surgery for chronic subdural hematoma. World Neurosurg. 129:e695–e699. 2019.

20. Sim YW, Min KS, Lee MS, Kim YG, Kim DH. Recent changes in risk factors of chronic subdural hematoma. J Korean Neurosurg Soc. 52:234–239. 2012.

21. Wada M, Yamakami I, Higuchi Y, Tanaka M, Suda S, Ono J, et al. Influence of antiplatelet therapy on postoperative recurrence of chronic subdural hematoma: a multicenter retrospective study in 719 patients. Clin Neurol Neurosurg. 120:49–54. 2014.

22. Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. 74:937–943. 2003.

24. Younsi A, Riemann L, Habel C, Fischer J, Beynon C, Unterberg AW, et al. Relevance of comorbidities and antithrombotic medication as risk factors for reoperation in patients with chronic subdural hematoma. Neurosurg Rev. 45:729–739. 2022.

25. Yuksel MO, Cevik S, Erdogan B, Tunckale T, Katar S, Isik S, et al. Effect of antithrombotic therapy on development of acute subdural hematoma after burr hole drainage of chronic subdural hematoma. Turk Neurosurg. 30:758–762. 2020.
Fig. 1.
The interval between discontinuation of medication and surgery by medication group. AP : antiplatelet agents, AC : anticoagulants.
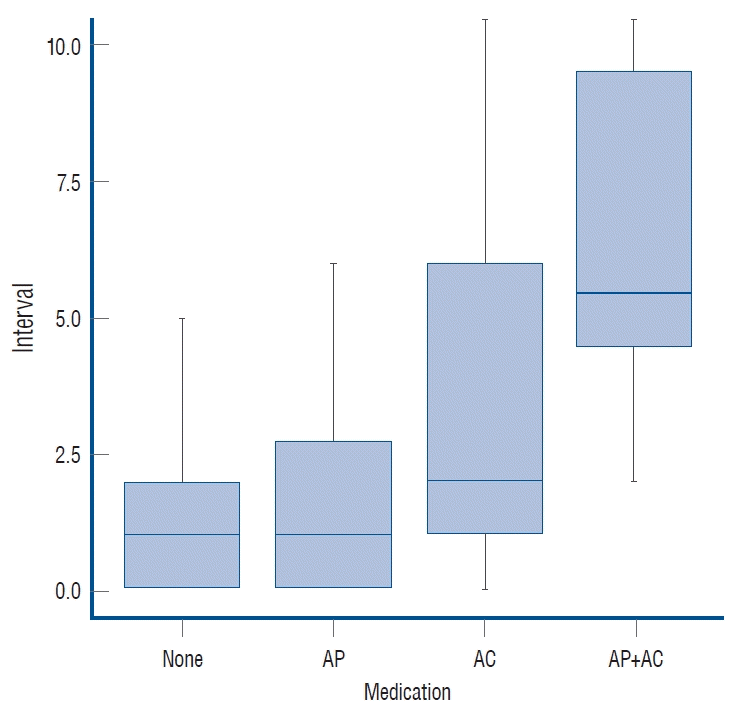
Fig. 2.
Duration of postoperative in-dwelling drainage catheter by medication group. AP : antiplatelet agents, AC : anticoagulants.
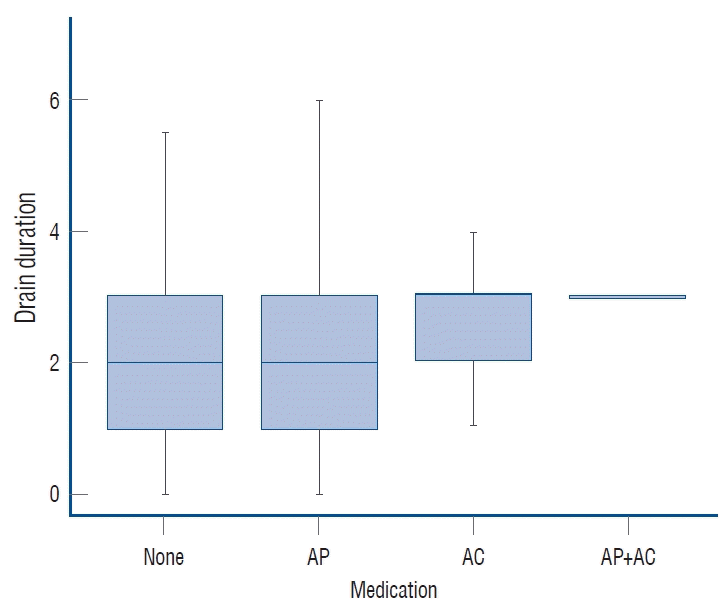
Table 1.
Baseline characteristics
Table 2.
Medication history of antiplatelet agents and anticoagulants
Table 3.
Clinical characteristics according to medication history of antiplatelet agents and anticoagulant
| No (n=343) | AP (n=100) | AC (n=17) | AP+AC (n=2) | p-value | |
|---|---|---|---|---|---|
| Timing of surgery | 1.00 (0.00–2.00) | 1.00 (0.00–2.750) | 2.0 (1.00–6.00) | 7.0 (4.5–9.5) | 0.318* |
| 0.048† | |||||
| Drain duration | 2.00 (1.00–3.00) | 2.00 (1.00–3.00) | 3.00 (2.00–3.00) | 3.00 (3.00–3.00) | 0.026* |
| 0.037† | |||||
| Acute hemorrhage on postoperative CT | 74 (21.57) | 19 (19.00) | 6(35.29) | 2 (100.00) | 0.808* |
| 0.044† | |||||
| Recurrence | 61 (17.78) | 12 (12.00) | 1 (5.88) | 2 (100.00) | 0.517* |
| 1.000† | |||||
| Reoperation | 49 (14.29) | 12 (12.00) | 1 (5.88) | 1 (50.00) | 0.924* |
| 1.000† | |||||
| Morbidity | 15 (4.37) | 5 (5.00) | 1 (5.88) | 1 (50.00) | 0.763* |
| 0.800† | |||||
| Mortality | 7 (2.04) | 3 (3.00) | 0 (0.00) | 0 (0.00) | 0.748* |
| 0.992† |
Table 4.
Review of previous articles concerning the relationship between AT and clinical course of postoperative CSDH patients
| Study | N | Result | |
|---|---|---|---|
| Effect of AT use on postoperative clinical features | |||
| Wada et al. [21] (2014) | 681 | More frequent recurrence of CSDH | |
| AP 92 | |||
| Fornebo et al. [7] (2017) | 763 | No effect on recurrence | |
| AT 308 | Higher morbidity | ||
| Abboud et al. [1] (2018) | 201 | No effect on recurrence | |
| AP 41 | |||
| Yuksel et al. [25] (2020) | 117 | No effect on acute hemorrhage | |
| AP 49 AC 36 | |||
| Zhang et al. [27] (2020) | 546 | Larger preoperative hematoma volume in AT | |
| AT 124 | Higher recurrence rate in the AC group | ||
| AP 81 AC 43 | No effect on recurrence (AP) | ||
| Choi et al. [5] (2020) | 230 | No effect on recurrence (univariate) | |
| AP 30 AC 6 | Increased odds ratio for recurrence (multivariate) | ||
| Younsi et al. [24] (2022) | 654 | No effect on reoperation, mortality | |
| AT 318 | |||
| Kerttula et al. [15] (2022) | 301 | No effect on recurrence | |
| AT 164 | |||
| Postoperative resumption of AT | |||
| Guha et al. [10] (2016) | 479 | AT patients had more frequent thromboembolic complications | |
| AT 231 | Recommend early resumption of AT | ||
| AP 164 AC 86 | |||
| Amano et al. [2] (2016) | 150 | AT patients had more frequent thromboembolic complications | |
| AT 44 | Recommend early resuming AT | ||
| Fornebo et al. [7] (2017) | 763 | Early resumption - No association with recurrence and lower thromboembolic complications | |
| AT 308 | Recommend early resumption of AT | ||
| Zhang et al. [26] (2021) | 621 | Time of resumption of AT - No effect on recurrence | |
| AT 139 | AT patients had more frequent thromboembolic complications | ||
| AP 104 AC 29 | Recommend early resuming AT | ||
| AP+AC 6 | |||
| Kerttula et al. [15] (2022) | 301 | More recurrence in early resumption group | |
| AT 164 | |||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download