Abstract
Nephrotic syndrome (NS) is associated with cerebral venous sinus thrombosis (CVST), which is a rare cerebrovascular disorder in children. Systemic anticoagulation with heparin is the standard therapy for CVST, and mechanical thrombectomy (MT) has been described as a salvage treatment for adult anticoagulant refractory CVST, However, it has never been reported in children. We describe a case of MT for refractory CVST in a child with NS. A 13-year-old boy with newly diagnosed NS presented to an emergency department with acute headache. A head computed tomography showed acute thrombus in the superior sagittal sinus, straight sinus and transverse sinus. The child was started on heparin therapy, but clinically deteriorated and became unresponsive. In view of the rapid deterioration of the condition after anticoagulation treatment, the patient received intravascular treatment. Several endovascular technologies, such as stent retriever and large bore suction catheter have been adopted. After endovascular treatment, the patient’s neurological condition was improved within 24 hours, and magnetic resonance venography of the head demonstrated that the CVST was reduced. The child recovered with normal neurological function at discharge. This case highlights the importance of considering MT for refractory CVST, and we suggest that MT may be considered for refractory CVST with NS in children.
Cerebral venous sinus thrombosis (CVST) is a rare cerebrovascular disorder with an incidence of seven cases per 1 million children per year [13,15]. Nephrotic syndrome (NS) is a clinical syndrome of proteinuria, edema, hypoproteinemia and hyperlipidemia, that has an incidence of 2–7 per 100000 children [14]. NS causes a hypercoagulable state, and one of the most serious complications of NS is thromboembolism. The incidence of thromboembolism in pediatric patients with NS is 2–5%. CVST is an unusual but fatal complication in patients with NS [7].
Anticoagulation is the current mainstay therapy for CVST, and despite treatment with systemic anticoagulation after admission, more than 23% of children die [4]. It have reported in a limited number of cases that endovascular thrombolysis treatment for CVST were superior to those with low-molecular weight heparin (LMWH) [10]. Mechanical thrombectomy (MT) has been proven to be more efficient than intravascular thrombolysis [5], and the primary goal of MT is to reduce the burden of clots, which may result in better penetration of the anticoagulant and improved venous drainage [12]. Based on the effectiveness of MT for CVST in adults, we report a case of MT treatment for CVTS in children with NS. MT treatment has never been reported in the pediatric neurosurgical literature, which may be due to a lack of clinician familiarity and the lack of neurologists that treat children, leading to underdiagnosis and insufficient management.
A 13-year-old boy was referred to our hospital with headache, dizziness and vomiting. He presented with mild edema of the eyelids and legs that was present for a month, and the patient was previously diagnosed with NS at the pediatric department of our hospital. He was treated with prednisone and tacrolimus. One week before admission, he was discharged from the pediatric department of our hospital. The patient complained of a frontal headache 3 days before admission, and the headache improved after the patient took nonsteroidal anti-inflammatory and analgesic drugs. The headache worsened 2 days before admission. Additionally, the child presented with dizziness and vomiting 1 day before admission.
On admission to the hospital, neurological examination showed symmetry, and there were no other pathologic findings except for pitting edema of his lower extremities and periorbital edema. His Glasgow coma scale (GCS) was 15. The tension, reflexes and strength of the peripheral extremities were normal. Cranial nerve examination was normal, and his cardiovascular and respiratory examinations were normal.
The initial observations on his physical examination were normal, with a blood pressure of 118/75 mmHg, a heart rate 65, a temperature of 36.6°C and the presence of lower leg edema. Urinalysis showed an elevated 24-hour urine protein quantification of 4.445 g/24 hours, urine microalbumin 562.2 mg/L (reference values, 0 to 60.2 mg/L), and urine microalbumin/urinary creatinine 528.9 mg/gcr (reference values, 0 to 32.3 mg/gcr). Meanwhile, coagulation test revealed an elevated D-dimer level (2.89 µg/mL), a fibrinogen level of 5.2 g/L, and decreased antithrombin III levels (37%), which usually decreases due to NS. Blood chemistry and serology showed the following complete blood count revealed a white blood cell count of 15.87×109/L, hemoglobin level of 172 g/L, and platelet count of 208×109/L. Blood chemistry and serology showed the following : elevated serum creatinine level of 73.3 µmol/L, a glomerular filtration rate of 151.6 mL/min. A low-density lipoprotein cholesterol level of 6.80 mmol/L, and a reduced serum albumin level of 13.7 g/L, which revealed that the patient’ s NS had relapsed. The hemophilic thrombus screening tests for hyperhomocysteinemia, antiphospholipid syndrome, thyroid disease, and systemic lupus erythematosus were normal, but the patient’s protein S level was decreased by 63% and his protein C level was increased by more than 200%.
On the day of admission, a head computed tomography (CT) revealed acute thrombosis of the superior sagittal sinus, right transverse sinus and the sigmoid sinus (Fig. 1A-C). The patient received bolus injections of enoxaparin sodium at a dose of 100 units/kg twice a day. A follow-up head CT showed that the thrombosis of the superior sagittal sinus and the right transverse sinus was unchanged from the previous scan. On the third day after admission, the patient’s consciousness level rapidly decreased. Magnetic resonance venography at that time showed thrombosis of the superior sagittal sinus and the right transverse sinus (Fig. 1D and E), venous dilatation in the corresponding area of the susceptibility weighted imaging (SWI) sequence (Fig. 1F), but no intracranial hemorrhage or limited diffusion dilatation. Digital subtraction angiography showed thrombus in the superior sagittal sinus and right transverse sinus (Fig. 1G and H), while no thrombus was found in the left transverse sinus (Fig. 1I). Therefore, we performed MT on this child with refractory CVST.
Because this patient was a child, we used the contrast agents and radiation very carefully. High-quality noninvasive imaging was performed to evaluate the venous anatomy. We punctured the femoral vein and artery at the same time, and only the key angiogram was performed. An 8 F 90 cm guiding catheter (Cordis, FL, USA) was positioned through the right common femoral vein in the distal right internal jugular vein (Fig. 2A and B). A rebar 18 catheter coaxially placed in the 072 skysurfer aspiration catheter (Skynor Medical, Shanghai, China) was navigated into the superior sagittal sinus using a 0.014″ shapeable guidewire (Fathom; Boston Scientific Corporation, Natick, MA, USA) (Fig. 2D). After contrast injection, a straight sinus thrombosis with impairment of outflow was shown (Fig. 2C). Remove Fathom guidewire, a 6×35 mm Syphonet stent retriever (Achieva Medical Limited, Suzhou, China) was deployed within the proximal straight sinus for 5 minutes (Fig. 2E). Thestent retriever was then removed by continuous aspiration through the Skysurfer aspiration catheter. Post thrombectomy angiography showed no significant improvement in venous drainage from the straight sinus to the transverse sinus. There was still no obvious improvement after two passes. After that, it was expanded by a 6×30 mm Sterling balloon dilatation catheter (Boston Scientific Corporation) to disrupt the thrombus (Fig. 2F), and then was deployed by a Syphonet stent retriever again for 5 minutes. It was removed again under continuous suction. After that, angiography demonstrated that the venous drainage was significantly improved, but there was a residual part of the clot (Fig. 2G-I). During the procedure, less than 30 mL of blood was collected.
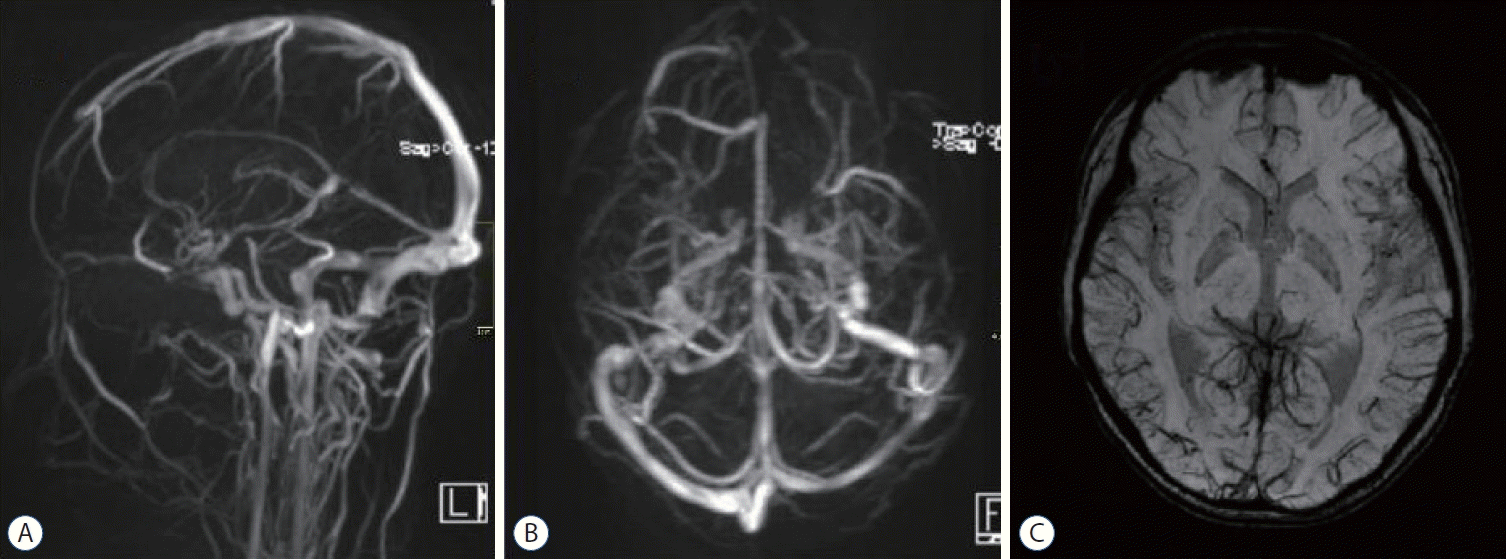
A head CT after the thrombectomy demonstrated a decrease in the previously seen hyperattenuation in the superior sagittal sinus and the right straight sinus (Fig. 3A-C). Histological analysis of the clot composition by Hematoxylin and Eosin staining revealed a mixed clot (Fig. 3D). The child continued to take enoxaparin twice a day after the thrombectomy. The patient’s neurological condition improved rapidly, and his GCS score was 15 on the second day after the thrombectomy. However, a lymphatic fistula of the right femoral vein puncture appeared, the lymphatic fistula closed after 2 days of compression, and the puncture point was scabbed over when the patient was discharged (Fig. 3E and F). The patient’s kidney function was monitored several times after the interventional therapy, and no significant changes were found. He was discharged from the hospital 8 days late with a completely normal neurologic examination. The patient took oral rivaroxaban an anticoagulant, daily. A follow-up magnetic resonance venography was performed 2 weeks after the endovascular therapy and demonstrated resolution of the thrombus, and the left and right sides of the cortical veins were symmetrical on the SWI sequence (Fig. 4). Three months after the onset of NS and CVT, the patient had no headaches.
NS is a recognized state of hypercoagulability, and children are at risk of venous thromboembolism, such as CSVT, pulmonary embolism, and renal vein thrombosis. Venous thromboembolism complicates 3% of NS cases in childhood. The pathophysiology of CSVT in NS is multifactorial and is caused by abnormal platelet aggregation, altered fibrinolysis, urinary loss of anticoagulant proteins (antithrombin III, proteins C and S), increased synthesis of prethrombotic factors (factors V and VIII), and intravascular fluid depletion. The most common but least specific symptom of CVST is headache, which is present in more than 90% of cases. It is the only symptom reported in 25% of patients [1]. Headache can be a characteristic of cerebral venous sinus obstruction at any location, but it is most prominent when there is a large cerebral sinus thrombus. Headache syndrome is an obvious feature of increased intracranial pressure, and this feature suggests focal lesions, ranging from edema following cortical vein occlusion to large vessel occlusion with massive infarction or hemorrhage [10]. Up to 50% of patients experience focal brain injury, such as ischemia, hemorrhage, or epilepsy [4]. In addition, severe patients present with rapid cognitive deterioration, which results in a depressed level of consciousness and coma; however, coma is a predictor of death in childhood CSVT [11]. The prognosis of children is worse than that of adults, and 20–70% of children show residual neurological defects.
CVST is a rare but potentially morbid and fatal disease in the pediatric population. The most obvious treatment option is anticoagulation with LMWH to prevent propagation of the thrombus and reduce the likelihood of complications, such as pulmonary embolus [1]. However, there is not enough evidence to suggest that LMWH is universally warranted to prevent venous thromboembolism in children with NS. The best management of the disease is not fully understood. Limited case reports and small case series of intervention local thrombolysis (urokinase, or recombinant tissue plasminogen activator [rt-PA]) and MT for CVST have been published. This therapy should be reserved for patients who present with very serious symptoms or have a rapid decline in neurological symptoms despite suitable anticoagulant treatment [2]. A meta-analysis [12] has shown the safety of intravascular therapy for CVST. That study included literature published from 1994 to 2014 and included a wide range of technologies and devices that have been used to treat patients, and that study demonstrated that the selection criteria for patients potentially benefiting from intravascular therapy are still not completely clear and that the treatment method lacks consensus. In a recent multicenter study [3], new generation, large bore suction catheters alone or in combination with stentriever devices were deployed for the treatment of CVST. All patients obtained good recanalization, none of the patients had major peri-procedural complications, and most of the patients were discharged to home or rehabilitation facilities. These findings suggest that endovascular treatment of adult CVST is a salvage therapy for anticoagulation refractory CVST cases. Data from the adult series show that more than 90% of patients with intravascular therapy can achieve favorable outcomes. However, there are limited reports on the application of endovascular treatment for CVST in pediatric patients. Mortimer et al. [9] reported using endovascular therapy in nine children with clinical deterioration. They used several endovascular methods, including intrusions infusion of rt-PA, aspiration, balloon angioplasty, and catheter disruption. In eight of the nine patients, good results were obtained. Gadgil et al. [6] reported seven children with CVST in five pediatric centers. Although these patients received systemic anticoagulation therapy, they received endovascular therapy due to neurological deterioration. Six of the seven patients had favorable outcomes. These studies suggest that endovascular therapy by experienced interventionalists may be safe and effective for children with severe CVST who fail frontline therapy treatment. In our case, the thrombus load was too large. In addition to the use of balloon thrombus disruption technology, we also used new generation, large bore suction catheters and stentriever devices to clear the thrombus.
Our study had several limitations. First, NS patients have renal insufficiency, and are at the risk of contrast-induced nephropathy (CIN). CIN is a common complication after cerebrovascular interventional diagnosis or therapeuty, and CIN may proceed to acute renal failure and is associated with significant mortality and morbidity [8]. Risk factors include older age, the use of high osmolar contrast media, previous renal dysfunction, such as NS. No strategies have been shown to effectively prevent CIN beyond thorough minimization of the amount of contrast agent, meticulous hydration of the patient, and patient selection. Our patient did not develop the complications of CIN, which may be related to the use of isotonic contrast agents and the minimization of the amount of contrast agent by use of double-arm DSA. However, we should still be alert to the complication of CIN in NS patients. Second, this case reports the off-label use of large bore suction catheters and stentriever devices to treat CVST. The current neurovascular techniques and devices are not specifically designed for the treatment of CVST, and most of the techniques and devices used for endovascular treatment of CVST are for the treatment of acute ischemic stroke. Therefore, further innovation and new devices specially designed for CVST and even for CVST in children are urgently needed. Endovascular intervention should be considered in a timely manner in children with radiographic or clinical progression despite anticoagulation.
In conclusion, we concluded that MT should not be ruled out in the treatment of refractory CVST in children with NS. It is urgent to carry out clinical trials of thrombectomy devices specially designed for CVST in children, and the results of these studies will be helpful for treatment decision-making.
Notes
References
1. Behrouzi R, Punter M. Diagnosis and management of cerebral venous thrombosis. Clin Med (Lond). 18:75–79. 2018.

2. Capecchi M, Abbattista M, Martinelli I. Cerebral venous sinus thrombosis. J Thromb Haemost. 16:1918–1931. 2018.

3. Dandapat S, Samaniego EA, Szeder V, Siddiqui FM, Duckwiler GR, Kiddy U, et al. Safety and efficacy of the use of large bore intermediate suction catheters alone or in combination for the treatment of acute cerebral venous sinus thrombosis: a multicenter experience. Interv Neuroradiol. 26:26–32. 2020.

4. Ferriero DM, Fullerton HJ, Bernard TJ, Billinghurst L, Daniels SR, DeBaun MR, et al. Management of stroke in neonates and children: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 50:e51–e96. 2019.

6. Gadgil N, Aldave G, Whitehead WE, Dmytriw AA, Chen K, Orbach D, et al. Endovascular intervention for refractory pediatric cerebral venous sinus thrombosis. Pediatr Neurol. 121:45–50. 2021.

7. Gao X, Liu Y, He Q, Shen X. Cerebral venous sinus thrombosis in nephrotic syndrome. Kidney Int. 101:1303. 2022.

8. Liu S, Shan XG, Zhang XJ. The place of hydration using intravenous fluid in patients at risk of developing contrast-associated nephropathy. Br J Hosp Med (Lond). 81:1–7. 2020.

9. Mortimer AM, Bradley MD, O’Leary S, Renowden SA. Endovascular treatment of children with cerebral venous sinus thrombosis: a case series. Pediatr Neurol. 49:305–312. 2013.

11. Sébire G, Tabarki B, Saunders DE, Leroy I, Liesner R, Saint-Martin C, et al. Cerebral venous sinus thrombosis in children: risk factors, presentation, diagnosis and outcome. Brain. 128:477–489. 2005.

12. Siddiqui FM, Dandapat S, Banerjee C, Zuurbier SM, Johnson M, Stam J, et al. Mechanical thrombectomy in cerebral venous thrombosis: systematic review of 185 cases. Stroke. 46:1263–1268. 2015.

Fig. 1.
A-C : Computed tomography head revealing a hyperdense sign noted (white arrows) in the superior sagittal sinus and transverse sinus suggesting cerebral venous sinus thrombosis. D and E : Magnetic resonance venography revealed thrombus in superior sagittal sinus and transverse sinus (white arrows). F : The right cortical veins (green arrows) on the susceptibility weighted imaging sequence are significantly larger than the left, suggesting obstruction of venous return. G and H : The venous phase of right internal carotid angiography suggests thrombosis of the superior sagittal and right transverse sinuses (white arrows). I : Venous phase of left internal carotid angiography. No thrombus was found in the left transverse sinus.
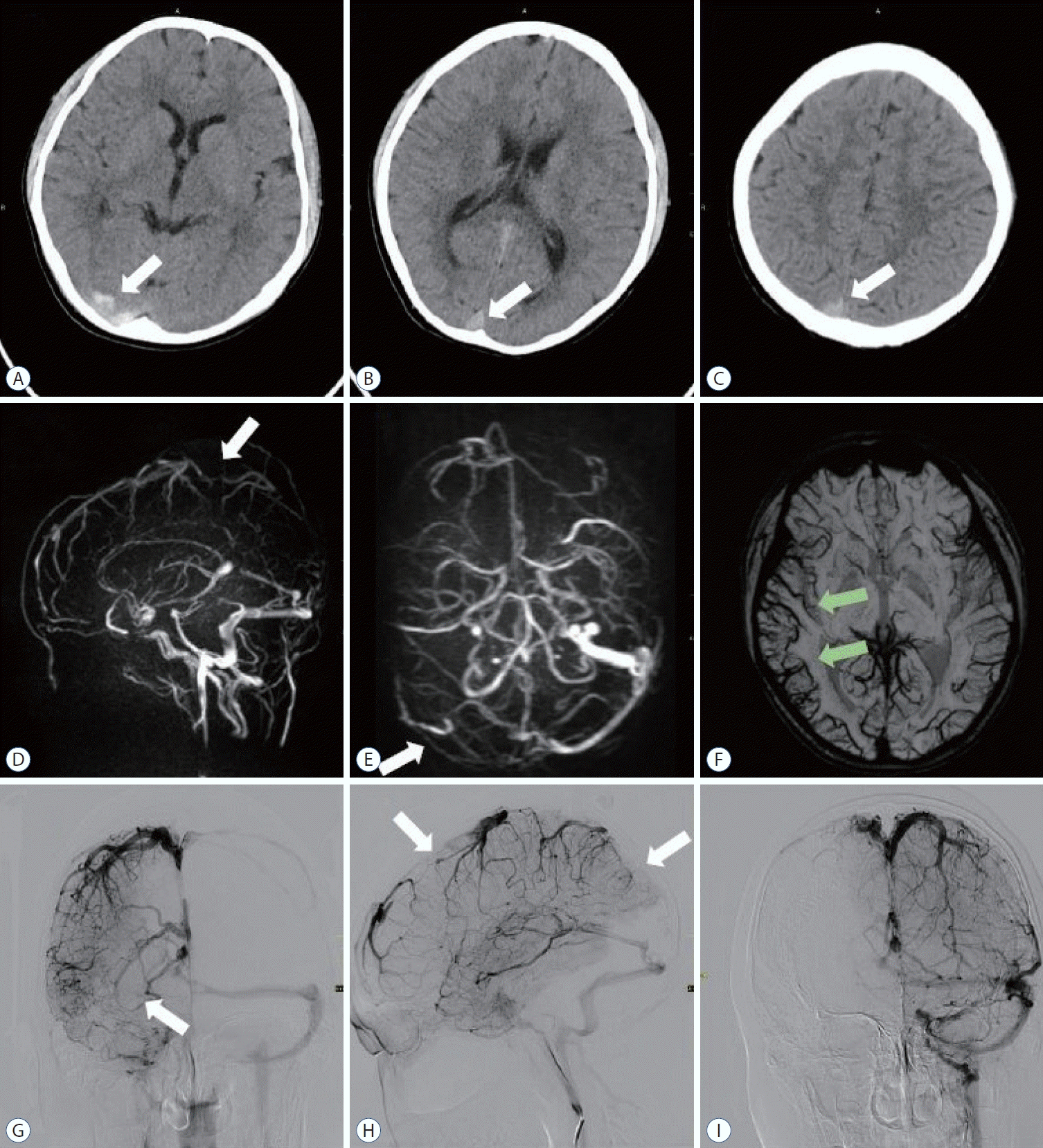
Fig. 2.
Procedure of transvenous endovascular mechanical thrombectomy. A and B : Right femoral vein approach, suction catheter guided target vessel reading. C : Intras sagittal sinus angiography suggests obstruction of venous return (white arrows). D-F : Large volume venous clots retrieved using the skysurfer aspiration catheter (white arrow), Syphonet stent retriever (green arrow), and balloon catheter disruption of the thrombus (black arrow). G-I : Right transverse sinus recanalization (white arrow), with a small amount of thrombus remaining (black arrow). Urokinase was administered into the superior sagittal and right transverse sinus. Anticoagulation with heparin was resumed after the procedure.
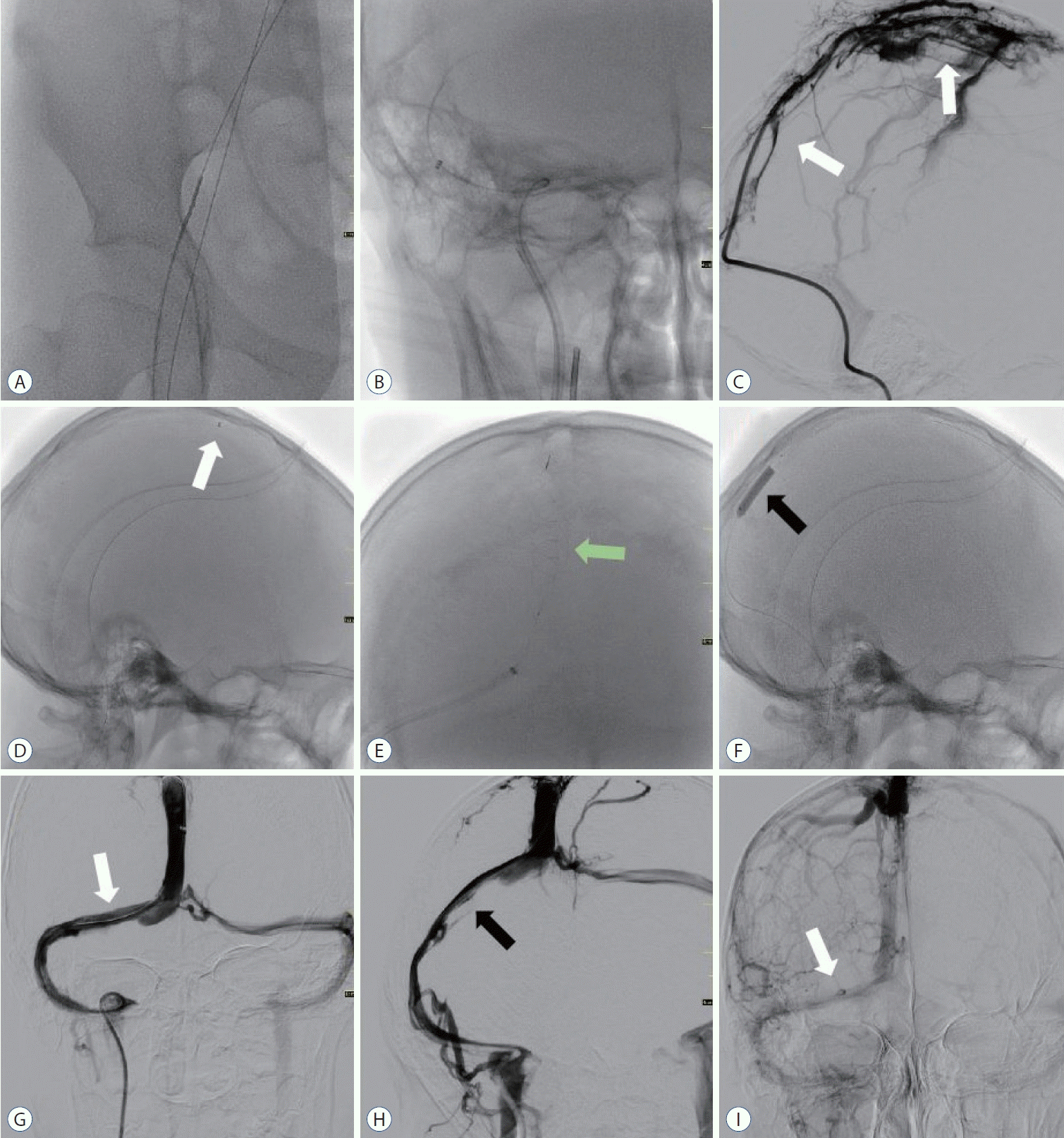
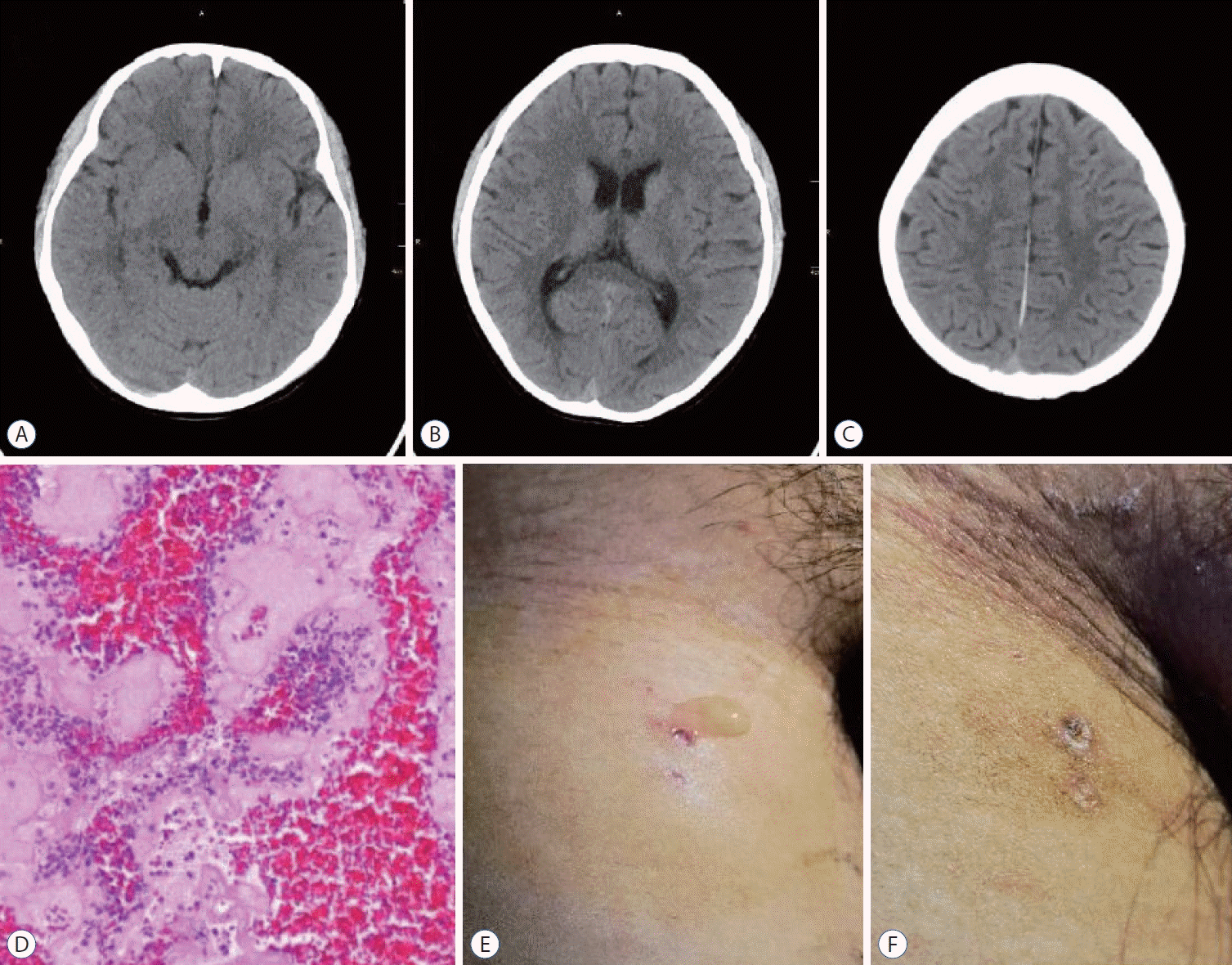
Fig. 3.
A-C : Computed tomography head revealing that the hyperdense sign had disappeared in the superior sagittal sinus and transverse sinus whitin 1 weak after endovascular mechanical thrombectomy. D : Hematoxylin and Eosin staining (×400) demonstrated that the cerebral venous sinus thrombosis was mixed clot. E and F : Lymphatic fluid oozes from the right femoral vein puncture point, stopped oozing after compression.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download