Abstract
Sweroside is a natural monoterpene derived from Swertia pseudochinensis Hara. Recently, studies have shown that sweroside exhibits a variety of biological activities, such as anti-inflammatory, antioxidant, and hypoglycemic effects. However, its role and mechanisms in high glucose (HG)-induced renal injury remain unclear. Herein, we established a renal injury model in vitro by inducing human renal tubular epithelial cell (HK-2 cells) injury by HG. Then, the effects of sweroside on HK-2 cell activity, inflammation, reactive oxygen species (ROS) production, and epithelial mesenchymal transition (EMT) were observed. As a result, sweroside treatment ameliorated the viability, inhibited the secretion of inflammatory cytokines (TNF-α, IL-1β, and VCAM-1), reduced the generation of ROS, and inhibited EMT in HK-2 cells. Moreover, the protein expression of SIRT1 was increased and the acetylation of p65 NF-kB was decreased in HK-2 cells with sweroside treatment. More importantly, EX527, an inhibitor of SIRT1, that inactivated SIRT1, abolished the improvement effects of sweroside on HK-2 cells. Our findings suggested that sweroside may mitigate HG-caused injury in HK-2 cells by promoting SIRT1-mediated deacetylation of p65 NF-kB.
Diabetes is becoming more prevalent worldwide, and in which diabetic nephropathy (DN), one of its major microvascular complications, has emerged as a pressing public health concern on a global scale [1,2]. The main etiology of DN are hyperglycemia, inflammatory transmitters, oxidative stress, and cytokine infiltration, and improperly controlled DN can progress to end-stage renal disease, threatening the life of patients [3-5]. Previous studies have shown that renal tubular involvement and renal tubular epithelial cell injury in high glucose (HG) contributed to early DN and DN development [6,7]. Hence, finding new drugs to protect against the damage of renal tubular epithelial cells in HG is necessary for the prevention and treatment of DN.
Sweroside is consisted of many bioactive Chinese medicinal materials such as Lonicera japonica, Gentiana, and Swertia mileensis [8]. It has been verified that sweroside exerts extensive pharmacological activities, including hypoglycemic, anti-inflammatory, anti-oxidant, and cardiovascular and cerebrovascular protection [9]. For example, sweroside played an anti-inflammatory role in LPS-stimulated acute lung damage in mice by activating silent information regulator 2 homolog 1 (SIRT1) [10], and prevented myocardial ischemia-reperfusion injury by mitigating oxidative stress and pyroptosis through regulating Keap1/Nrf2 axis [11]. In addition, sweroside can be therapeutic for hepatitis and liver fibrosis [8,12]. Yang et al. [13] determined that sweroside, which acted as an inhibitor for NLRP3, decreased the levels of interleukin (IL)-1β in the hepatic, which beneficially devoted to improving non-alcoholic steatohepatitis. However, the effects of sweroside on renal injury induced by HG are still unclear.
In this study, in vitro renal injury model was established by inducing human renal tubular epithelial cell (HK-2 cells) damage by HG. Thus, we explored the role of sweroside in diabetic kidney injury and the potential downstream mechanism at cell levels.
HK-2 cells were obtained from ATCC. Cells were seeded in DMEM/F-12 medium (Gibco) containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin, and then grown in an incubator (37°C, 5% CO2). With about 80% confluency, the mediums were replaced with mediums containing normal glucose (NG, 5.5 mM) or HG (30 mM) for 48 h, respectively. Before HG treatment, cells were pre-treated with sweroside (CAS no. 14215-86-2; purity ≥ 98%) and EX527 (Sigma-Aldrich) at indicated concentrations for 30 min.
The cell viability of HK-2 cells was detected by CCK8 (Beyotime). In brief, after the required treatment, cells were co-incubated with CCK8 (10 µl) for another 2 h. Then, the absorbance was recorded at 450 nm with a microplate reader (Thermo Labsystems). The cell viability was calculated as the ratio of the mean absorbance value of the treatment group/control group.
Following treatment, the HK-2 cells were harvested, washed with PBS, and suspended in Annexin V binding buffer. The cell supernatant was then stained with 5 µl of Annexin-V-FITC and 15 µl of propidium iodide solution. The apoptotic cells were determined using flow cytometry.
The total RNA from HK-2 cells was extracted using Trizol reagent (Invitrogen), and then reverse-transcribed into cDNA. Subsequently, qRT-PCR was conducted utilizing the SYBR Green methods (Vazyme) on the ViiA 7 real-time PCR instrument (Life Technologies).
Cell culture supernatants were harvested, and the concentration of tumor necrosis factor-α (TNF-α), IL-1β, and VCAM-1 were measured through ELISA assays using TNF-α ELISA kit (ml077385), IL-1β ELISA kit (ml058059) and VCAM-1 ELISA kit (ml060757) from Enzyme-linked Biotechnology, respectively.
The ROS production in HK-2 cells with different treatments was measured by DHE staining (Beyotime). Briefly, cells were preprocessed with or without sweroside and EX527 and then exposed to HG. Next, DHE (5 µM) was mixed with each sample and incubated for an additional 30 min at 37°C. The fluorescence images were observed and captured with a fluorescence microscope (IX73; OLYMPUS).
After the required treatment, cells were harvested and then lysed with RIPA solution (Beyotime). Following denaturing at 95°C, protein samples were separated by SDS-PAGE and transferred to the PVDF membrane. Next, the membrane was co-incubated with anti-ZO-1 (ab216880, 1:1,000, Abcam), anti-Vimentin (ab16700, 1:100, Abcam), anti-αSMA (ab88979, 1:500, Abcam), anti-Snail (ab216347, 1:1,000, Abcam), anti-SIRT1 (ab110304, 1:1,000, Abcam), anti-Ac-p65 (ab19870, 1:500, Abcam), anti-p65 (ab16502, 1:1,000, Abcam) and anti-β-actin (ab8227, 1:1,000, Abcam) primary antibodies, and subsequently co-incubated with corresponding secondary antibodies. Protein bands were developed using an ECL kit (Sigma-Aldrich) and then analyzed by Image J software.
All assays above were conducted no less than three times. GraphPad Prism 8.0 (GraphPad Software) was utilized for statistical analysis. Data were shown as mean ± SD. Differences between two groups were determined using a t-test, and differences among multiple groups were analyzed by one-way ANOVA. p < 0.05 was regarded as significant.
Sweroside (chemical structure shown in Fig. 1A) is a natural monoterpene and belongs to iridoid glucoside [8]. To explore the effect of sweroside on HG-treated HK-2 cells viability, we first evaluated the activity of cells treated with sweroside (0, 25, 50, 100 µM) under non-HG conditions. As a result, different concentrations of sweroside had no marked impact on cell viability without HG (Fig. 1B). Next, CCK8 assay was performed under HG stimulation. The results showed that cell viability was observably decreased in the HG group compared to the control group. Moreover, the HG + SW (50 µM) and HG + SW (100 µM) groups had enhanced activity of cells compared with the HG group, but no significant difference was observed between the HG + SW (25 µM) group and the HG group (Fig. 1C). Subsequently, the result of flow cytometry showed that the rate of cell apoptosis was increased when cells were exposed to HG, whereas sweroside protected cells from HG-induced apoptosis (Fig. 1D, E). Taken together, these data revealed that sweroside alleviated the decreased viability and increased apoptosis of HK-2 cell under HG stimulation.
Next, the levels of inflammatory factors including TNF-α, IL-1β, and VCAM-1 were analyzed by qRT-PCR and ELISA to assess the effect of sweroside on the cellular inflammatory response induced by HG. As shown in Fig. 2A, B, in comparison with the control group, TNF-α, IL-1β, and VCAM-1 mRNA levels, and their concentrations in the culture medium were markedly increased in the HG group. In addition, the levels of TNF-α, IL-1β, and VCAM-1 were decreased in HG + SW (50, 100 µM) group compared to the HG group. Furthermore, DHE fluorescent staining was conducted to measure the level of intracellular ROS. The results showed that exposure of HK-2 cells to HG led to an enhancement in DHE fluorescence as compared to the control group. As expected, the enhancement of DHE fluorescence upon HG exposure was eliminated by sweroside (Fig. 2C). These data suggested that sweroside could alleviated HG-induced inflammation response and oxidative injury in HK-2 cells.
The result of the Western blot showed that the protein level of epithelial cell markers (ZO-1) was dramatically reduced, while interstitial cell markers (Vimentin, α-SMA, and Snail) were increased in the HG group as compared to the control group. Furthermore, compared with the HG group, expression of ZO-1 was notably elevated and Vimentin, α-SMA, and Snail were decreased in HG + SW (25, 50, 100 µM) groups (Fig. 3). These data demonstrated that sweroside inhibited the EMT in HK-2 cells induced by HG.
A further study showed that HG treatment inhibited the protein level of SIRT1 in HK-2 cells. By contrast, a prominently increased expression of SIRT1 was observed in HG-exposed HK-2 cells after sweroside (100 µM) treatment, but no significant change was observed after sweroside (25 and 50 µM) treatment (Fig. 4A). Subsequently, we tested the acetylation level of p65 NF-kB, and the results showed that HG exposure increased p65 acetylation level and that sweroside (25, 50, 100 µM) treatment notably decreased HG-induced p65 acetylation in HK-2 cells (Fig. 4B). These data suggested that sweroside may deacetylate p65 by activating SIRT1, which resulted in downregulation of NF-kB pathway activity.
Finally, EX527, an inhibitor of SIRT1, was used to definite the implication of SIRT1 in the positive contribution of sweroside in HK-2 cells injury. Western blot showed that SIRT1 expression was reduced in HG + SW (100 µM) + EX527 group in comparison to the HG + SW (100 µM) group (Fig. 5A). The detection of cell viability, inflammation, and oxidative stress showed that the promotion of cell activity and the inhibition of TNF-α, IL-1β, and VCAM-1 levels of sweroside on HG-treated HK-2 cells were reversed by EX527, meanwhile, the weakening effect of sweroside on HG-induced oxidative stress in HK-2 cells was impeded by EX527 (Fig. 5B-D). In addition, compared with the HG + SW (100 µM) group, the protein expression of ZO-1 was reduced and Vimentin, α-SMA, and Snail levels were increased in HG + SW (100 µM) + EX527 group (Fig. 5E). Together, these data indicated that the effect of sweroside was reversed by EX527 treatment in HG-injured HK-2 cells.
DN is a microvascular complication of diabetes, which is related to local inflammation of the kidney [14]. Moreover, it is generally considered that oxidative stress is the basic pathogenesis of diabetes complications [15]. Excessive ROS in diabetes could lead to oxidative injury in intrinsic cells of the kidney, thus activating those signaling pathways linked to DN [16]. Renal tubular epithelial cells, as the main target for DN, showed to be injured at the early stage of DN [7,17]. Therefore, drugs that relieve the damage of renal tubular epithelial cells may contribute to blocking DN progression. Herein, we analyzed the effects of sweroside on cell survival, inflammation, and oxidative injury of HK-2 cells induced by HG, and found that sweroside protected HK-2 cells from HG-induced damage.
Renal interstitial fibrosis is a common pathological pathway for chronic kidney disease to progress to end-stage renal failure, while renal tubular EMT is a significant process of renal interstitial fibrosis [18,19]. Therefore, in chronic kidney injury, targeted inhibition of EMT in renal tubular epithelial cells is a potential antifibrotic treatment that can aid in reversing nephropathy. Herein, we confirmed that the protein level of tight junction protein ZO-1 was decreased and fibroblast marker proteins including Vimentin, α-SMA, and Snail were increased in HG-induced HK-2 cells. These results were consistent with previously published work reported by Mu et al. [20] and Gong et al. [21]. Moreover, Wang et al. [22] found that Shenkang injection could alleviate tubulopathy in the DN rat model by inhibiting EMT. Zang et al. [23] revealed that the dysfunction of renal and EMT of glomerular was attenuated in DN rat model treatment with Icariin. In our study, we found sweroside reversed the EMT in HK-2 cells induced by HG, suggesting it may be an effective therapeutic agent for DN. However, its clinical application value needs further verification.
SIRT1 is a NAD-dependent protein deacetylase that has been proven to be renal protective [24]. Herein, we found that sweroside could prominently promote SIRT1 protein expression in HK-2 cells exposed to HG. As reported, SIRT1 played a beneficial effect on DN [25]. For example, Qiu et al. [26] verified that up-regulation of SIRT1 could block the oxidative injury and apoptosis of tubular in DN. Du et al. [27] indicated that SIRT1 mediated YY1 deacetylation to reverse renal tubular EMT in DN. Moreover, we confirmed that sweroside could inhibit the acetylation of p65 NF-kB in HG-induced HK-2 cells. As an important nuclear transcription factor, NF-kB is activated by various stimuli, such as hyperglycemia, obesity, and oxidative stress [28,29]. The evidence derived from a large amount of assays has shown that NF-kB was activated in the kidney of DN rats, and its inhibition helps to improve DN [30,31]. At present, the vital role of the SIRT1/NF-kB pathway in DN has been acknowledged [32,33]. Our results suggested that sweroside may exert its protective role in DN by regulating SIRT1/NF-kB signaling pathway. To confirm this speculation, a SIRT1 inhibitor EX527 was used, and the results showed that the effects of sweroside on HK-2 cells under HG condition such as the promotion of cell activity and the inhibition of inflammatory factors secretion, ROS production, and EMT of were reversed by EX527. As expected, the acetylation level of p65 was increased by EX527 treatment in HK-2 cells.
In conclusion, our results definitely indicated that sweroside could alleviate the injury of HK-2 cells exposed to HG by up-regulating SIRT1 expression and mediating deacetylation of p65 NF-kB, suggesting a potential kidney-protective effect of sweroside in DN.
REFERENCES
1. Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, Zhou M, Tang X, Hu Y, Wang L. 2017; Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 317:2515–2523. DOI: 10.1001/jama.2017.7596. PMID: 28655017. PMCID: PMC5815077.

2. Vahid H, Rakhshandeh H, Ghorbani A. 2017; Antidiabetic properties of Capparis spinosa L. and its components. Biomed Pharmacother. 92:293–302. DOI: 10.1016/j.biopha.2017.05.082. PMID: 28551550.

3. Chi K, Geng X, Liu C, Cai G, Hong Q. 2020; Research progress on the role of inflammasomes in kidney disease. Mediators Inflamm. 2020:8032797. DOI: 10.1155/2020/8032797. PMID: 32410864. PMCID: PMC7204206. PMID: 310b2e9c3709471997608d226f173bf7.

4. Masood S, Rehman AU, Bashir S, El Shazly M, Imran M, Khalil P, Ifthikar F, Jaffar HM, Khursheed T. 2021; Investigation of the anti-hyperglycemic and antioxidant effects of wheat bread supplemented with onion peel extract and onion powder in diabetic rats. J Diabetes Metab Disord. 20:485–495. DOI: 10.1007/s40200-021-00770-x. PMID: 34222073. PMCID: PMC8212200.

5. Samsu N. 2021; Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. 2021:1497449. DOI: 10.1155/2021/1497449. PMID: 34307650. PMCID: PMC8285185.

6. Zheng C, Huang L, Luo W, Yu W, Hu X, Guan X, Cai Y, Zou C, Yin H, Xu Z, Liang G, Wang Y. 2019; Inhibition of STAT3 in tubular epithelial cells prevents kidney fibrosis and nephropathy in STZ-induced diabetic mice. Cell Death Dis. 10:848. DOI: 10.1038/s41419-019-2085-0. PMID: 31699972. PMCID: PMC6838321.

7. Liu L, Bai F, Song H, Xiao R, Wang Y, Yang H, Ren X, Li S, Gao L, Ma C, Yang X, Liang X. 2022; Upregulation of TIPE1 in tubular epithelial cell aggravates diabetic nephropathy by disrupting PHB2 mediated mitophagy. Redox Biol. 50:102260. Erratum in: Redox Biol. 2022;52:102302. DOI: 10.1016/j.redox.2022.102302. PMID: 35365434. PMCID: PMC9108084.

8. Gong J, Yang F, Yang Q, Tang X, Shu F, Xu L, Wang Z, Yang L. 2020; Sweroside ameliorated carbon tetrachloride (CCl4)-induced liver fibrosis through FXR-miR-29a signaling pathway. J Nat Med. 74:17–25. DOI: 10.1007/s11418-019-01334-3. PMID: 31280460.

9. Nuntawong P, Horikawa T, Tanaka H, Morimoto S, Sakamoto S. 2022; Activated carbon-based immunochromatographic strip test for the rapid qualitative analysis of swertiamarin and sweroside. J AOAC Int. 105:1460–1467. DOI: 10.1093/jaoacint/qsac054. PMID: 35521980.

10. Wang J, Cai X, Ma R, Lei D, Pan X, Wang F. 2021; Anti-inflammatory effects of sweroside on LPS-induced ALI in mice via activating SIRT1. Inflammation. 44:1961–1968. DOI: 10.1007/s10753-021-01473-4. PMID: 33913051.

11. Li J, Zhao C, Zhu Q, Wang Y, Li G, Li X, Li Y, Wu N, Ma C. 2021; Sweroside protects against myocardial ischemia-reperfusion injury by inhibiting oxidative stress and pyroptosis partially via modulation of the Keap1/Nrf2 axis. Front Cardiovasc Med. 8:650368. DOI: 10.3389/fcvm.2021.650368. PMID: 33816579. PMCID: PMC8017130. PMID: dc69b42000b5434ba587be0b47535f43.

12. Wang R, Dong Z, Lan X, Liao Z, Chen M. 2019; Sweroside alleviated LPS-induced inflammation via SIRT1 mediating NF-κB and FOXO1 signaling pathways in RAW264.7 cells. Molecules. 24:872. DOI: 10.3390/molecules24050872. PMID: 30823686. PMCID: PMC6429084. PMID: d5f4aae37d7548d18d8ae4f76f5f4d0d.

13. Yang G, Jang JH, Kim SW, Han SH, Ma KH, Jang JK, Kang HC, Cho YY, Lee HS, Lee JY. 2020; Sweroside prevents non-alcoholic steatohepatitis by suppressing activation of the NLRP3 inflammasome. Int J Mol Sci. 21:2790. DOI: 10.3390/ijms21082790. PMID: 32316419. PMCID: PMC7216241. PMID: 8a50a65db41243f9a4ea0f916a0365c6.

14. Su WY, Li Y, Chen X, Li X, Wei H, Liu Z, Shen Q, Chen C, Wang YP, Li W. 2021; Ginsenoside Rh1 improves type 2 diabetic nephropathy through AMPK/PI3K/Akt-mediated inflammation and apoptosis signaling pathway. Am J Chin Med. 49:1215–1233. DOI: 10.1142/S0192415X21500580. PMID: 34049473.

15. Darenskaya MA, Kolesnikova LI, Kolesnikov SI. 2021; Oxidative stress: pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull Exp Biol Med. 171:179–189. DOI: 10.1007/s10517-021-05191-7. PMID: 34173093. PMCID: PMC8233182.

16. Østergaard JA, Cooper ME, Jandeleit-Dahm KAM. 2020; Targeting oxidative stress and anti-oxidant defence in diabetic kidney disease. J Nephrol. 33:917–929. DOI: 10.1007/s40620-020-00749-6. PMID: 32447617.

17. Jiang WJ, Xu CT, Du CL, Dong JH, Xu SB, Hu BF, Feng R, Zang DD, Meng XM, Huang C, Li J, Ma TT. 2022; Tubular epithelial cell-to-macrophage communication forms a negative feedback loop via extracellular vesicle transfer to promote renal inflammation and apoptosis in diabetic nephropathy. Theranostics. 12:324–339. DOI: 10.7150/thno.63735. PMID: 34987648. PMCID: PMC8690920.

18. Xu H, Wang M, Li Y, Shi M, Wang Z, Cao C, Hong Y, Hu B, Zhu H, Zhao Z, Chu X, Zhu F, Deng X, Wu J, Zhao F, Guo J, Wang Y, Pei G, Zhu F, Wang X, et al. 2022; Blocking connexin 43 and its promotion of ATP release from renal tubular epithelial cells ameliorates renal fibrosis. Cell Death Dis. 13:511. DOI: 10.1038/s41419-022-04910-w. PMID: 35641484. PMCID: PMC9156700. PMID: 6f6ee1751e934bffab9d0808415a38ad.

19. Sun Y, Cai H, Ge J, Shao F, Huang Z, Ding Z, Dong L, Chen J, Zhang J, Zang Y. 2022; Tubule-derived INHBB promotes interstitial fibroblast activation and renal fibrosis. J Pathol. 256:25–37. DOI: 10.1002/path.5798. PMID: 34543458.

20. Mu L, Chen N, Chen Y, Yang Z, Zhou H, Song S, Shi Y. 2022; Blocking REDD1/TXNIP complex ameliorates HG-induced renal tubular epithelial cell apoptosis and EMT through repressing oxidative stress. Int J Endocrinol. 2022:6073911. DOI: 10.1155/2022/6073911. PMID: 36186658. PMCID: PMC9519289. PMID: fa5785edd79349fba71e38cee71f5761.

21. Gong EY, Jo HA, Park SH, Cha DR, Hur DY, Han SY. 2020; VSIG4 induces epithelial-mesenchymal transition of renal tubular cells under high-glucose conditions. Life (Basel). 10:354. DOI: 10.3390/life10120354. PMID: 33348749. PMCID: PMC7766063. PMID: 2b8591792cf54a8996235a2f6d1b95e0.

22. Wang WW, Liu YL, Wang MZ, Li H, Liu BH, Tu Y, Yuan CC, Fang QJ, Chen JX, Wang J, Fu Y, Wan ZY, Wan YG, Wu W. 2021; Inhibition of renal tubular epithelial mesenchymal transition and endoplasmic reticulum stress-induced apoptosis with Shenkang injection attenuates diabetic tubulopathy. Front Pharmacol. 12:662706. DOI: 10.3389/fphar.2021.662706. PMID: 34408650. PMCID: PMC8367077. PMID: 6e5a17a3b367498cb8b8c6b3e2d83cd2.

23. Zang L, Gao F, Huang A, Zhang Y, Luo Y, Chen L, Mao N. 2022; Icariin inhibits epithelial mesenchymal transition of renal tubular epithelial cells via regulating the miR-122-5p/FOXP2 axis in diabetic nephropathy rats. J Pharmacol Sci. 148:204–213. DOI: 10.1016/j.jphs.2021.10.002. PMID: 35063135.

24. Morigi M, Perico L, Benigni A. 2018; Sirtuins in renal health and disease. J Am Soc Nephrol. 29:1799–1809. DOI: 10.1681/ASN.2017111218. PMID: 29712732. PMCID: PMC6050939.

25. Yan J, Wang J, He JC, Zhong Y. 2022; Sirtuin 1 in chronic kidney disease and therapeutic potential of targeting Sirtuin 1. Front Endocrinol (Lausanne). 13:917773. DOI: 10.3389/fendo.2022.917773. PMID: 35795148. PMCID: PMC9251114. PMID: dd1232cc2b4042e495d9c1e72fc56a1b.

26. Qiu D, Song S, Wang Y, Bian Y, Wu M, Wu H, Shi Y, Duan H. 2022; NAD(P)H: quinone oxidoreductase 1 attenuates oxidative stress and apoptosis by regulating Sirt1 in diabetic nephropathy. J Transl Med. 20:44. DOI: 10.1186/s12967-021-03197-3. PMID: 35090502. PMCID: PMC8796493. PMID: b38ee5a4e12d48039ab077a30b16484f.

27. Du L, Qian X, Li Y, Li XZ, He LL, Xu L, Liu YQ, Li CC, Ma P, Shu FL, Lu Q, Yin XX. 2021; Sirt1 inhibits renal tubular cell epithelial-mesenchymal transition through YY1 deacetylation in diabetic nephropathy. Acta Pharmacol Sin. 42:242–251. DOI: 10.1038/s41401-020-0450-2. PMID: 32555442. PMCID: PMC8027604.

28. Shen J, Dai Z, Li Y, Zhu H, Zhao L. 2022; TLR9 regulates NLRP3 inflammasome activation via the NF-kB signaling pathway in diabetic nephropathy. Diabetol Metab Syndr. 14:26. DOI: 10.1186/s13098-021-00780-y. PMID: 35120573. PMCID: PMC8815223. PMID: bda1900c32394c89a7ce7548fac756cd.

29. Tao Y, Lin Y, An L, Tao Y, Li Y, Han J, Hu W, Liu W. 2022; Knockdown of GPRC5B alleviates the high glucose-induced inflammation and extracellular matrix deposition of podocyte through inhibiting NF-κB pathway. Allergol Immunopathol (Madr). 50:142–146. DOI: 10.15586/aei.v50i2.566. PMID: 35257557.

30. Fu Y, Wang X, Zhang L, Ren Y, Hao L. 2022; Allograft inflammatory factor-1 enhances inflammation and oxidative stress via the NF-κB pathway in diabetic kidney disease. Biochem Biophys Res Commun. 614:63–69. DOI: 10.1016/j.bbrc.2022.04.089. PMID: 35569377.

31. Zhang Y, Chen X, Fan Y, Liu J, Yuan L. 2022; XCL1 aggravates diabetic nephropathy-mediated renal glomerular endothelial cell apoptosis and inflammatory response via regulating p53/nuclear factor-kappa B pathway. Nephron. 146:84–98. DOI: 10.1159/000518172. PMID: 34518457.

32. Liu Y, Liu W, Zhang Z, Hu Y, Zhang X, Sun Y, Lei Q, Sun D, Liu T, Fan Y, Li H, Ding W, Fang J. 2021; Yishen capsule promotes podocyte autophagy through regulating SIRT1/NF-κB signaling pathway to improve diabetic nephropathy. Ren Fail. 43:128–140. DOI: 10.1080/0886022X.2020.1869043. PMID: 33427556. PMCID: PMC7808384. PMID: 8893498db4184214915f16bd0f2dddbe.

33. Sun HJ, Xiong SP, Cao X, Cao L, Zhu MY, Wu ZY, Bian JS. 2021; Polysulfide-mediated sulfhydration of SIRT1 prevents diabetic nephropathy by suppressing phosphorylation and acetylation of p65 NF-κB and STAT3. Redox Biol. 38:101813. DOI: 10.1016/j.redox.2020.101813. PMID: 33279869. PMCID: PMC7718489.

Fig. 1
Sweroside promotes the cell viability of HK-2 cells exposed to HG.
(A) The chemical structure of sweroside. (B) The activity of cells treated with sweroside (0, 25, 50, 100 µM) under non-HG conditions were determined by CCK8 assay. (C) CCK8 assay was performed under HG exposure. (D, E) Flow cytometry analysis of cell apoptosis under HG exposure. Values are presented as mean ± SD. SW, sweroside; HK-2 cells, human renal tubular epithelial cell; HG, high glucose; Ctrl, control. NSp > 0.05, compared with non-SW group or HG group; ***p < 0.001, compared with the Ctrl group; ###p < 0.001, compared with the HG group.
Fig. 2
Sweroside attenuates HG-induced inflammation and oxidative stress in HK-2 cells.
(A) The mRNA levels of TNF-α, IL-1β, and VCAM-1 were detected by qRT-PCR. (B) The concentrations of TNF-α, IL-1β, and VCAM-1 in the culture medium were examined by ELISA. (C) Representative images of DHE fluorescent staining showing ROS production. Scale bar = 50 µM. Values are presented as mean ± SD. SW, sweroside; HK-2 cells, human renal tubular epithelial cell; HG, high glucose; Ctrl, control; TNF-α, tumor necrosis factor-α; IL, interleukin; qRT-PCR, quantitative real-time polymerase chain reaction; ROS, reactive oxygen species. ***p < 0.001, compared with the Ctrl group; NSp > 0.05, #p < 0.05, ##p < 0.01, ###p < 0.001, compared with the HG group.
Fig. 3
Sweroside reverses EMT-related proteins expression in HK-2 cells under HG condition.
(A) Blot images were gained from the Western blot membrane. (B) Quantification of the Western blot assay. Values are presented as mean ± SD. SW, sweroside; HK-2 cells, human renal tubular epithelial cell; HG, high glucose; Ctrl, control; EMT, epithelial mesenchymal transition. ***p < 0.001, compared with the Ctrl group; ##p < 0.01, ###p < 0.001, compared with the HG group.
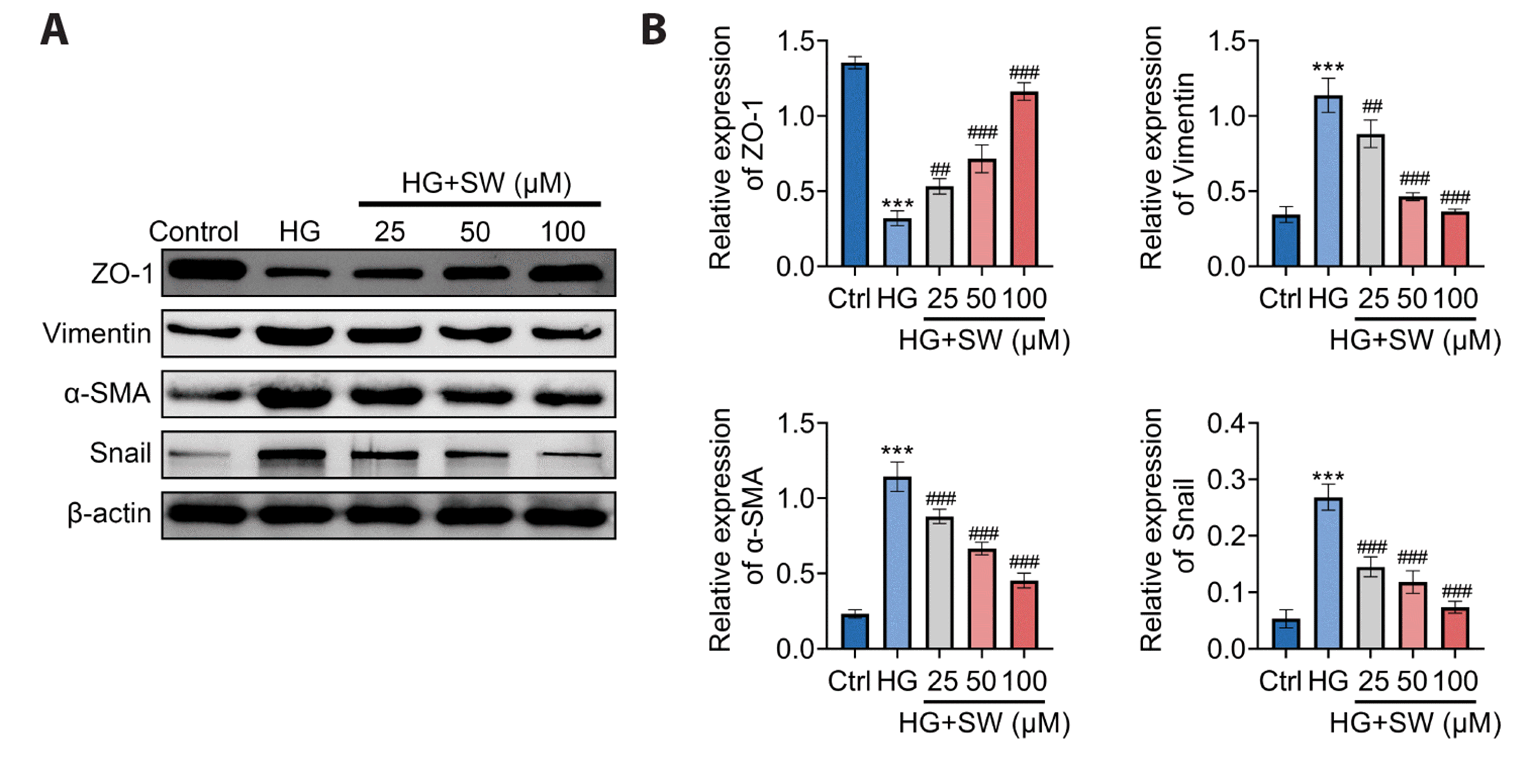
Fig. 4
Sweroside regulates SIRT1/NF-kB signaling pathway in HG-induced HK-2 cells.
(A) SIRT1 protein level in HK-2 cells under HG condition was examined by Western blot. (B) Ac-p65, p65, and β-actin protein expression were detected by Western blot. Values are presented as mean ± SD. SW, sweroside; HK-2 cells, human renal tubular epithelial cell; HG, high glucose; Ctrl, control; SIRT1, silent information regulator 2 homolog 1. ***p < 0.001, compared with the Ctrl group; NSp > 0.05, ###p < 0.001, compared with the HG group.
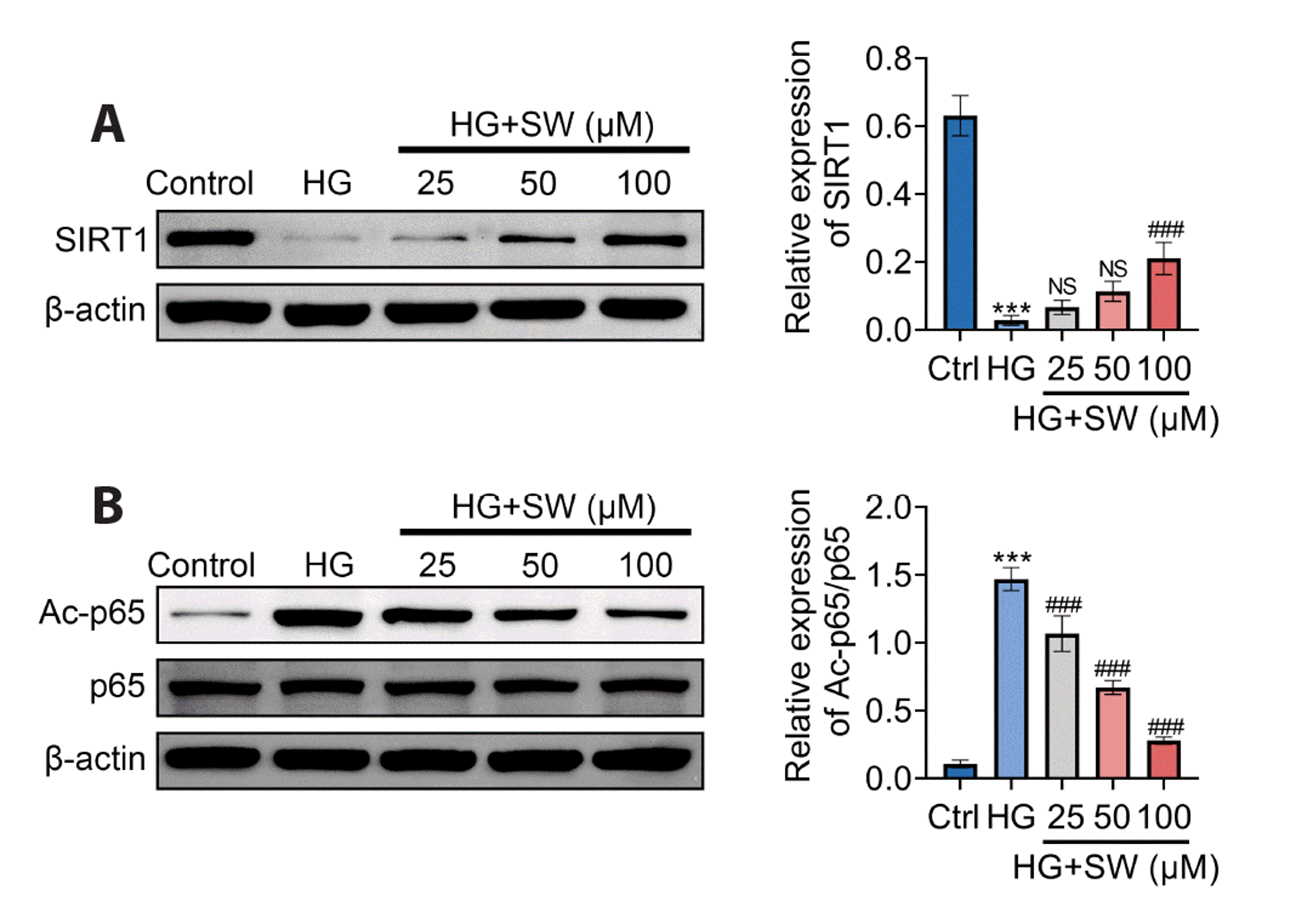
Fig. 5
EX527 reversed the alleviating effects of sweroside on HG-treated HK-2 cells.
(A) Western blot assay for SIRT1 protein expression in HK-2 cells of Ctrl group, HG group, HG + SW (100 µM) group and HG + SW (100 µM) + EX527 group. (B) CCK8 assay for cell viability. (C) ELISA was performed to analyze TNF-α, IL-1β, and VCAM-1 concentration. (D) Detection of ROS in different groups of HK-2 cells by DHE fluorescent staining. Scale bar = 50 µM. (E) Western blot assays for ZO-1, Vimentin, α-SMA, and Snail protein expression. Values are presented as mean ± SD. SW, sweroside; HK-2 cells, human renal tubular epithelial cell; HG, high glucose; Ctrl, control; SIRT1, silent information regulator 2 homolog 1; TNF-α, tumor necrosis factor-α; IL, interleukin; ROS, reactive oxygen species. ***p < 0.001, compared with the control group; ##p < 0.01, ###p < 0.001, compared with the HG group; ^^p < 0.01, ^^^p < 0.001, compared with the HG + SW (100 µM) group.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download