Abstract
Transmembrane protein TMEM16A, which encodes calcium-activated chloride channel has been implicated in tumorigenesis. Overexpression of TMEM16A is associated with poor prognosis and low overall survival in multiple cancers including lung adenocarcinoma, making it a promising biomarker and therapeutic target. In this study, three structure-related sesquiterpene lactones (mecheliolide, costunolide and dehydrocostus lactone) were extracted from the traditional Chinese medicine Aucklandiae Radix and identified as novel TMEM16A inhibitors with comparable inhibitory effects. Their effects on the proliferation and migration of lung adenocarcinoma cells were examined. Whole-cell patch clamp experiments showed that these sesquiterpene lactones potently inhibited recombinant TMEM16A currents in a concentration-dependent manner. The half-maximal concentration (IC50) values for three tested sesquiterpene lactones were 29.9 ± 1.1 μM, 19.7 ± 0.4 μM, and 24.5 ± 2.1 μM, while the maximal effect (Emax) values were 100.0% ± 2.8%, 85.8% ± 0.9%, and 88.3% ± 4.6%, respectively. These sesquiterpene lactones also significantly inhibited the endogenous TMEM16A currents and proliferation, and migration of LA795 lung cancer cells. These results demonstrate that mecheliolide, costunolide and dehydrocostus lactone are novel TMEM16A inhibitors and potential candidates for lung adenocarcinoma therapy.
Ca2+-activated Cl- channels (CaCCs) are ion channels that are widely expressed in various organs and are involved in many physiological processes, such as humoral secretion, smooth muscle contraction, neuronal excitation, and tumor development [1,2]. Transmembrane protein TMEM16A, also known as Anoctamin1, is a principal component of the CaCCs. The tmem16a gene is located within chromosome 11q13 and is frequently amplified in various types of tumors [3,4].
Overexpression of TMEM16A is associated with many cancers, including head and neck squamous cell carcinoma, oral cancer, salivary gland cancer, esophageal squamous cell carcinoma, breast cancer, lung cancer, etc. [5-8]. Lung cancer has received increasing attention due to its high incidence and mortality rate among TMEM16A-associated cancers. Global Cancer Statistics report show that lung cancer accounts for 11.6% of all cancers worldwide and has a mortality rate of 18.4% [9]. Several studies have shown that the evolution of lung adenocarcinoma is influenced by TMEM16A [10]. For instance, it was reported that TMEM16A was highly expressed in the lung adenocarcinoma cell line, LA795 [11]. These reports suggest that downregulation or inhibition of TMEM16A expression can effectively suppress the growth of cancer [6,11,12].
In lung adenocarcinoma or other cancer types with high expression levels of TMEM16A, potent and highly selective inhibitors of TMEM16A may exert therapeutic benefits. Several compounds, such as CaCCinh-A01, T16Ainh-A01, and MONNA, have been identified as inhibitors of TMEM16A [13-15]. However, most of these inhibitors are chemically synthesized compounds, and have several disadvantages such as low potency and poor selectivity. Therefore, researchers have identified safe and effective active compounds from natural products as novel TMEM16A inhibitors, such as Cepharanthine [16] and Matrine [11].
Aucklandiae Radix (AR) is the dried root of Aucklandia lappa Decne and is commonly used as a traditional Chinese herbal medicine. It improves the movement of qi to relieve pain, fortifies the spleen and promotes digestion [17]. Modern pharmacological studies have revealed the anticancer effects of the main active components of AR, such as costunolide and dehydrocostus lactone [17,18]. By screening 530 natural product collections by utilizing BrdU assay and patch clamp technique in LA795 lung adenocarcinoma cells [19], we identified three active sesquiterpene lactones effectively inhibited TMEM16A currents. Moreover, our results also provide evidence that TMEM16A inhibition using sesquiterpene lactones of AR may be responsible for their anticancer effects.
mTMEM16A cDNA (GenBank No. NM_178642.5) were provided by Prof. Uhtaek Oh (Seoul National University). The human TMEM16B (NM_001364562) cDNA clones were purchased from Youbio Biological Technology Co., Ltd. Chinese hamster ovary (CHO) cell lines stably transfected with mTMEM16A were provided by Prof. Hailin Zhang (Hebei Medical University). LA795 cells were sourced from the Cell Bank of the Chinese Academy of Sciences.
Nonessential amino acids (1%) and 600 μg/ml G418 were added to F-12K (Solarbio) to culture CHO cells stably transfected with TMEM16A. LA795 cells were cultured in RPMI 1640 (Gibco). All media were supplemented with 10% fetal bovine serum (FBS, Gibco) and antibiotics (100 IU/ml penicillin G and 100 mg/ml streptomycin; Solarbio). The cells were seeded on 24-multiwell plates with glass coverslips and maintained at 37°C in a humidified atmosphere with 5% CO2 and 95% air. Patch-clamp technique was performed on the cells within 12 h to 48 h of seeding.
CHOK cells were cultured in F-12K (Solarbio) and seeded on glass coverslips and transient transfected with TMEM16B and EGFP expression plasmids using X-tremeGENE 9 DNA Transfection Reagent (Roche) according to the manufacturer’s manual. Patch clamp recordings were made 24 h after cells were transfected, and cells were used within 48 h.
Whole-cell patch clamp technique was used to record cell currents of CHO or LA795 cells in an air-conditioned room (23°C–25°C). Pipette were pulled out from a borosilicate glass capillary with resistance in the range of 2–3 MΩ and injected into the electrode fluid. Axon patch 200B or 700B amplifier and pClamp 10.6 software (Axon Instruments) were used to record currents at 5 kHz and filtered at 2 kHz. The external solution used to record TMEM16A currents contained 160 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 8 mM glucose, and a pH of 7.4. The pipette solution contained 130 mM CsCl, 10 mM EGTA, 1 mM MgCl2, 10 mM HEPES, 2 mM ATP, and a pH of 7.3 (with different concentration of CaCl2). Free Ca2+ concentration was calculated using CaEGTA Calculator V1.2 (https://somapp.ucdmc.ucdavis.edu/pharmacology/bers/maxchelator/CaEGTA-NIST.htm). Whole-cell TMEM16A currents were recorded using voltage ramps from −80 mV to +80 mV over 4 sec. Drug effects quantified by measuring the change in current amplitude at +80 mV. Current-voltage (I–V) relationships were explored by setting step pulses between −80 mV and +80 mV, starting with a holding potential of 0 mV, which was stepped up to −100 mV, and then increased every 20 mV until a stable current was obtained for recording.
LA795 cells were seeded on 96-well plates for 24 h and then treated with different concentrations of mecheliolide, costunolide and dehydrocostus lactone (1, 3, 10, 30, 100 μM), or MONNA (1–300 μM) for 24 h; cells treated with dimethyl sulfoxide (DMSO) for 24 h served as the control. The CCK-8 reagent (500 μg/ml) was added to each well, and the cells were further cultured for 4 h following the manufacturer's instructions (AbMole BipScience). A microplate reader (Varioskan lux; Thermo Fisher Scientific, Inc.) was used to measure the absorbance of the sample at 450 nm. All proliferation tests were repeated thrice, and the proliferation value relative to control with 100% survival was calculated.
LA795 cells were seeded in trans-well 24 insert plate chambers (pore size: 8.0 μm; Corning), then coated with BioCoatMatrigel (BD Biosciences). The cells were pre-starved by culturing in serum-free medium for 18 h, and then seeded in the upper chambers filled with serum-free medium at a density of 3 × 104 LA795 cells per well. The lower chambers were filled with medium containing 10% FBS and different concentrations of mecheliolide, costunolide and dehydrocostus lactone (1, 3, 10, 30, 100 μM), or MONNA (1–300 μM) for 72 h. The cells that did not migrate were removed using a cotton swab. They were then fixed with 4% paraformaldehyde and stained with 0.5% crystal violet. The cells were counted using Image J software (National Institutes of Health).
Mecheliolide, costunolide and dehydrocostus lactone were obtained from Chengdu Despite Biotechnology Co., Ltd. MONNA was obtained from Sigma-Aldrich Corp. The compound collections used for screening included 530 natural products were purchased from TargetMol. The stock solutions were prepared in DMSO and stored at −20°C. All solutions were freshly prepared from stock solutions before each experiment and protected from light. The final concentration of DMSO was less than 0.1%.
The concentration-response curve was fitted using a logistic function: y = A2 + (A1 – A2) / [1 + (x / x0) p], where y and x represent the response and drug concentration, respectively. A1, A2, x0, and p represent maximum response, minimum response, IC50, and Hill coefficient, respectively. Data normalization was used to test the drug effects on the I-V relationships. Current amplitude was normalized by testing the current at +80 mV in the absence of the drugs to reduce the unwanted sources of variation.
A two-sample paired Student’s t-test or one-way ANOVA was used for statistical analyses. Data analysis, including graphic creation and statistical analysis, was performed using Origin 9.0 (Origin Lab Corp.). A p-value less than 0.05 was considered statistically significant. The data were expressed as mean ± SEM.
The whole-cell patch clamp recordings showed that three sesquiterpene lactones isolated from AR (mecheliolide, costunolide and dehydrocostus lactone) are potent TMEM16A inhibitors. The inhibitory effects of the three lactones were compared with those of MONNA, a known TMEM16A inhibitor. Their chemical structures are shown in Fig. 1. As shown in Supplementary Fig. 1 and Fig. 1, mecheliolide, costunolide and dehydrocostus lactone, share a similar sesquiterpene lactones structure with an ER Ca2+ releasing agent, thapsigargin (TG) (TG is an inhibitor of sarcoendoplasmic Ca2+-ATPase), all induced an intracellular Ca2+ transients in CHO cells at 30 μM.
The TMEM16A-mediated CaCCs were recorded in the whole-cell patch-clamp mode with 300 nM free Ca2+ in the pipette solution. A −80 mV to +80 mV voltage ramp occurring over 4 s was used to activate and record whole-cell recombinant TMEM16A currents in CHO cells (Fig. 2E). The effects of the three sesquiterpene lactones (100 μM) on the recombinant TMEM16A currents are shown in Fig. 2A–C and the results are summarized in Fig. 2F. Mecheliolide, costunolide and dehydrocostus lactone at 100 μM significantly inhibited TMEM16A currents at +80 mV, with inhibition effects of 88.5% ± 2.4%, 86.1% ± 2.8%, and 88.4% ± 3.4%, respectively. Application of 10 μM MONNA inhibited TMEM16A currents by 90.2% ± 1.5% at +80 mV (Fig. 2D). The inhibitory effects of the lactones are similar to that of MONNA.
TMEM16B is the closest analog of TMEM16A, so we have tested the effects of sesquiterpene lactones on TMEM16B currents expressed in CHO cells. As shown in Supplementary Fig. 2, mecheliolide (30 μM) did not affect the TMEM16B currents, while costunolide and dehydrocostus lactone (30 μM) significantly inhibited TMEM16B currents at +80 mV, with inhibition effects of 26.7% ± 4.8% and 63.4% ± 2.7%, respectively.
We further investigated the voltage dependency of the effects of mecheliolide, costunolide and dehydrocostus lactone on TMEM16A Cl− currents. In Fig. 3A, a 1.5-s voltage step protocol was used to elicit TMEM16A currents and I-V relationship curves were derived from voltage-dependent TMEM16A activation currents. The effects of mecheliolide, costunolide and dehydrocostus lactone at 100 μM each on the recombinant TMEM16A Cl− channels are shown in Fig. 3B, and summarized in Fig. 3C–E. The effect of MONNA, a small molecule inhibitor that blocks TMEM16A currents, was also tested for comparison.
Different concentrations of mecheliolide triggered a depolarization potential of +80 mV to cause a concentration-dependent inhibition of the recombinant TMEM16A currents. As shown in the Fig. 4A and B, mecheliolide (1, 3, 10, 30, and 100 μM) inhibited the TMEM16A currents in a concentration-dependent manner. The concentration-response curves of the three sesquiterpene lactones were fitted with the logistic function (Fig. 4C). The IC50 and Emax values of TMEM16A inhibition for mecheliolide, costunolide and dehydrocostus lactone were 29.9 ± 1.1 μM, 19.7 ± 0.4 μM, 24.5 ± 2.1 μM and 100.0% ± 2.8%, 85.8% ± 0.9%, 88.3% ± 4.6%, respectively (Fig. 4D).
Next, the inhibitory effects of mecheliolide, costunolide and dehydrocostus lactone on endogenous TMEM16A currents were explored. In our previous studies, we found that TMEM16A was highly expressed in LA795 lung adenocarcinoma cells [16] and recorded typical voltage and inward Ca2+ concentration-dependent TMEM16A currents (Fig. 5A). We used the LA795 cell lines to determine if mecheliolide, costunolide and dehydrocostus lactone inhibit endogenous TMEM16A currents. In the present study, the three sesquiterpene lactones significantly inhibited the endogenous TMEM16A currents in LA795 cells at +80 mV at a concentration of 100 μM (Fig. 5B, C). Notably, mecheliolide, costunolide and dehydrocostus lactone inhibited the currents by 89.6% ± 1.8%, 92.0% ± 1.5%, and 89.8% ± 1.7%, respectively. In comparison, MONNA (10 μM) induced 97.7% ± 2.1% inhibition on the currents (Fig. 5B, C).
CCK-8 and trans-well assays were performed to investigate the effects of different concentrations of mecheliolide, costunolide and dehydrocostus lactone on the proliferation and migration of LA795 cells. To explore the effects of the three sesquiterpene lactones on cell growth, we treated LA795 cells with 1–100 μM of the compounds for 24 h and observed the cells under an inverted microscope (Fig. 6A). As shown in Fig. 6B–D, the IC50 values of mecheliolide, costunolide and dehydrocostus lactone for inhibiting proliferation were 11.5 ± 2.1 μM, 9.8 ± 2.2 Μm, and 12.4 ± 3.0 μM, whereas the Emax values were 95.1% ± 1.8%, 93.6% ± 1.0%, and 93.7% ± 0.8%, respectively. The inhibitory effects of the three sesquiterpene lactones on cell proliferation were dose-dependent. Trans-well migration assay was performed to assess the effect of mecheliolide, costunolide and dehydrocostus lactone on LA795 cell migration. The cells were treated with different concentrations of mecheliolide, costunolide and dehydrocostus lactone for 72 h. Results showed that the number of migrating cells was reduced in a concentration-dependent manner. Compared with control cells, mecheliolide, costunolide and dehydrocostus lactone (10 μM) significantly inhibited migration through inhibition of TMEM16A (Fig. 7A). We then fitted the concentration-activity curves with a logistic function. The IC50 values of the migration inhibitory effects of mecheliolide, costunolide and dehydrocostus lactone were 12.1 ± 0.2 μM, 11.8 ± 1.8 μM, 10.3 ± 0.8 μM, and the Emax values were 100% ± 0.9%, 98.8% ± 2.4%, and 94.9% ± 2.8%, respectively (Fig. 7B–D).
The effects of MONNA (1–300 μM) on the cell proliferation and migration in LA795 cells were tested. MONNA concentration-dependently inhibited the proliferation and migration of LA795 cells, as compared with the control group (Fig. 6E, F and Fig. 7E, F). Based on the aforementioned results, we surmise that inhibition of endogenous TMEM16A mediated currents by these sesquiterpene lactones contributes to drugs associated anti-cancer effects in LA795 cells.
A growing number of studies suggests that TMEM16A-CaCCs are overexpressed in a variety of cancers and has been implicated in the regulation of tumorigenesis [20,21], suggesting that specific and potent TMEM16A inhibitors may be effective treatments for cancer [22]. In the current study, we have demonstrated, for the first time, that mecheliolide, costunolide and dehydrocostus lactone isolated from AR were potent TMEM16A-mediated CaCC inhibitors and that this inhibition may be responsible for their anticancer effects.
Using the patch clamp technique, three sesquiterpene lactones were found to inhibit TMEM16A currents. The IC50 values for mecheliolide-, costunolide-, and dehydrocostus-induced inhibition of TMEM16A were 29.9 ± 1.1 μM, 19.7 ± 0.4 μM, and 24.5 ± 2.1 μM, respectively. These inhibitory effects of the three compounds were more potent compared with many of the reported TMEM16A inhibitors. For instance, the reported IC50 values of conventional Cl− channel blockers are 150 μM for eugenol and 64.1 μM for 5-Nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB).
Data indicate that TMEM16A is endogenously overexpressed in approximately 77.3% lung adenocarcinoma patients or cell lines [22]. Additionally, there is evidence that several TMEM16A inhibitors, such as, arctigenin [12], matrine [11], and natural flavonoids [10] can efficiently reduce cell proliferation and migration in vitro and inhibit the growth of tumors in vivo.
A significant correlation occurred between TMEM16A inhibition and cell proliferation and migration reduction. These findings indicate that TMEM16A can serve as a potential therapeutic target for the treatment of lung adenocarcinoma. This was consistent with the results of this study. Mecheliolide, costunolide and dehydrocostus lactone can efficiently reduce endogenous TMEM16A-mediated currents, the migration and proliferation of LA975 cells. Overall, our study provides evidence that this sesquiterpene lactones are novel TMEM16A inhibitors and could be used in the treatment of lung adenocarcinoma.
AR is a commonly used Chinese herbal medicine that has gained significant attention due to its antitumor properties and safety [17]. Several chemical components of AR have been separated and identified, including terpenes, anthraquinones, flavonoid glycosides, shikokiols compounds and chlorogenic acid. Terpenoids are the main active constituents of AR. Terpenes mainly include eucalyptone type sesquiterpenes, sesquiterpenes, monoterpenes and triterpenes. Mecheliolide, costunolide and dehydrocostus lactone are the major sesquiterpene lactones of AR. Some studies showed that AR had antibacterial effects [23] and anti-inflammatory effects [24], which may explain the traditional use of AR in disease treatment. The anti-tumor effects of active components of AR such as costunolide and dehydrocostus lactone have also been reported. Tastan et al. [25] showed that costunolide and dehydrocostus lactone significantly inhibited the viability of MCF-7 and MDA-MB-453 cells. Choi et al. [26] demonstrated that costunolide is active in the inhibition of the 3-isobutyl-1-methylxanthine (IBMX)-induced melanogenesis in B-16 mouse melanoma cells. A recent study showed that mecheliolide elicited reactive oxygen species (ROS)-mediated endoplasmic reticulum stress (ERS) driven immunogenic cell death in hepatocellular carcinoma [27]. In the present study, we found that mecheliolide, costunolide and dehydrocostus lactone were potent TMEM16A inhibitors that inhibited the exogenous and endogenous TMEM16A-mediated CaCC currents. They also inhibited the proliferation and migration of LA795 cells. These findings provide new insight for designing TMEM16A inhibitors and reveal the anti-cancer mechanism of AR as a traditional Chinese medicine.
In conclusion, we found that mecheliolide, costunolide and dehydrocostus lactone effectively inhibited endogenous TMEM16A-mediated CaCC currents to induce anti-tumor effects in LA795 cells. The results indicated that natural TMEM16A inhibitors from AR can be used as potential treatments for lung adenocarcinoma or other cancers with high levels of TMEM16A expression. However, further detailed in vivo studies are needed to confirm the efficacy and safety of three sesquiterpene lactones isolated from AR before translation to the clinic.
Notes
SUPPLEMENTARY MATERIALS
Supplementary data including two figures can be found with this article online at https://doi.org/10.4196/kjpp.2023.27.6.521
REFERENCES
1. Huang F, Wong X, Jan LY. 2012; International Union of Basic and Clinical Pharmacology. LXXXV: calcium-activated chloride channels. Pharmacol Rev. 64:1–15. DOI: 10.1124/pr.111.005009. PMID: 22090471. PMCID: PMC3250081.

2. Scudieri P, Sondo E, Ferrera L, Galietta LJ. 2012; The anoctamin family: TMEM16A and TMEM16B as calcium-activated chloride channels. Exp Physiol. 97:177–183. DOI: 10.1113/expphysiol.2011.058198. PMID: 21984732.

3. Huang X, Gollin SM, Raja S, Godfrey TE. 2002; High-resolution mapping of the 11q13 amplicon and identification of a gene, TAOS1, that is amplified and overexpressed in oral cancer cells. Proc Natl Acad Sci U S A. 99:11369–11374. DOI: 10.1073/pnas.172285799. PMID: 12172009. PMCID: PMC123263.

4. Katoh M, Katoh M. 2003; FLJ10261 gene, located within the CCND1-EMS1 locus on human chromosome 11q13, encodes the eight-transmembrane protein homologous to C12orf3, C11orf25 and FLJ34272 gene products. Int J Oncol. 22:1375–1381. DOI: 10.3892/ijo.22.6.1375. PMID: 12739008.

5. Crottès D, Jan LY. 2019; The multifaceted role of TMEM16A in cancer. Cell Calcium. 82:102050. DOI: 10.1016/j.ceca.2019.06.004. PMID: 31279157. PMCID: PMC6711484.

6. Sui Y, Sun M, Wu F, Yang L, Di W, Zhang G, Zhong L, Ma Z, Zheng J, Fang X, Ma T. 2014; Inhibition of TMEM16A expression suppresses growth and invasion in human colorectal cancer cells. PLoS One. 9:e115443. DOI: 10.1371/journal.pone.0115443. PMID: 25541940. PMCID: PMC4277312. PMID: e1799d879dad4397a88da7567f62dfe4.

7. Shang L, Hao JJ, Zhao XK, He JZ, Shi ZZ, Liu HJ, Wu LF, Jiang YY, Shi F, Yang H, Zhang Y, Liu YZ, Zhang TT, Xu X, Cai Y, Jia XM, Li M, Zhan QM, Li EM, Wang LD, et al. 2016; ANO1 protein as a potential biomarker for esophageal cancer prognosis and precancerous lesion development prediction. Oncotarget. 7:24374–24382. DOI: 10.18632/oncotarget.8223. PMID: 27016410. PMCID: PMC5029708.

8. Wang H, Zou L, Ma K, Yu J, Wu H, Wei M, Xiao Q. 2017; Cell-specific mechanisms of TMEM16A Ca2+-activated chloride channel in cancer. Mol Cancer. 16:152. DOI: 10.1186/s12943-017-0720-x. PMID: 28893247. PMCID: PMC5594453. PMID: 98ad363855fd4819a4f8e9fc20e5474f.
9. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018; Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68:394–424. Erratum in: CA Cancer J Clin. 2020;70:313. DOI: 10.3322/caac.21492. PMID: 30207593.

10. Zhang X, Li H, Zhang H, Liu Y, Huo L, Jia Z, Xue Y, Sun X, Zhang W. 2017; Inhibition of transmembrane member 16A calcium-activated chloride channels by natural flavonoids contributes to flavonoid anticancer effects. Br J Pharmacol. 174:2334–2345. DOI: 10.1111/bph.13841. PMID: 28452066. PMCID: PMC5481650.

11. Guo S, Chen Y, Pang C, Wang X, Shi S, Zhang H, An H, Zhan Y. 2019; Matrine is a novel inhibitor of the TMEM16A chloride channel with antilung adenocarcinoma effects. J Cell Physiol. 234:8698–8708. DOI: 10.1002/jcp.27529. PMID: 30370542.

12. Guo S, Chen Y, Shi S, Wang X, Zhang H, Zhan Y, An H. 2020; Arctigenin, a novel TMEM16A inhibitor for lung adenocarcinoma therapy. Pharmacol Res. 155:104721. DOI: 10.1016/j.phrs.2020.104721. PMID: 32097750.

13. Boedtkjer DM, Kim S, Jensen AB, Matchkov VM, Andersson KE. 2015; New selective inhibitors of calcium-activated chloride channels - T16A(inh) -A01, CaCC(inh) -A01 and MONNA - what do they inhibit? Br J Pharmacol. 172:4158–4172. DOI: 10.1111/bph.13201. PMID: 26013995. PMCID: PMC4543620.

14. Seo Y, Lee HK, Park J, Jeon DK, Jo S, Jo M, Namkung W. 2016; Ani9, a novel potent small-molecule ANO1 inhibitor with negligible effect on ANO2. PLoS One. 11:e0155771. DOI: 10.1371/journal.pone.0155771. PMID: 27219012. PMCID: PMC4878759. PMID: 65ccc41fa1db43e88b5ab5b7aca30b52.

15. Oh SJ, Hwang SJ, Jung J, Yu K, Kim J, Choi JY, Hartzell HC, Roh EJ, Lee CJ. 2013; MONNA, a potent and selective blocker for transmembrane protein with unknown function 16/anoctamin-1. Mol Pharmacol. 84:726–735. DOI: 10.1124/mol.113.087502. PMID: 23997117. PMCID: PMC3807079.

16. Zhang X, Zhang G, Zhao Z, Xiu R, Jia J, Chen P, Liu Y, Wang Y, Yi J. 2021; Cepharanthine, a novel selective ANO1 inhibitor with potential for lung adenocarcinoma therapy. Biochim Biophys Acta Mol Cell Res. 1868:119132. DOI: 10.1016/j.bbamcr.2021.119132. PMID: 34450215.

17. Huang Z, Wei C, Yang K, Yu Z, Wang Z, Hu H. 2021; Aucklandiae Radix and Vladimiriae Radix: a systematic review in ethnopharmacology, phytochemistry and pharmacology. J Ethnopharmacol. 280:114372. DOI: 10.1016/j.jep.2021.114372. PMID: 34186101.

18. Zhuang K, Xia Q, Zhang S, Maharajan K, Liu K, Zhang Y. 2021; A comprehensive chemical and pharmacological review of three confusable Chinese herbal medicine-Aucklandiae radix, Vladimiriae radix, and Inulae radix. Phytother Res. 35:6655–6689. DOI: 10.1002/ptr.7250. PMID: 34431559.

19. Zhang X, Zhang G, Zhai W, Zhao Z, Wang S, Yi J. 2020; Inhibition of TMEM16A Ca2+-activated Cl- channels by avermectins is essential for their anticancer effects. Pharmacol Res. 156:104763. DOI: 10.1016/j.phrs.2020.104763. PMID: 32201246.
20. Luo S, Wang H, Bai L, Chen Y, Chen S, Gao K, Wang H, Wu S, Song H, Ma K, Liu M, Yao F, Fang Y, Xiao Q. 2021; Activation of TMEM16A Ca2+-activated Cl- channels by ROCK1/moesin promotes breast cancer metastasis. J Adv Res. 33:253–264. DOI: 10.1016/j.jare.2021.03.005. PMID: 34603794. PMCID: PMC8463928.

21. Saberbaghi T, Wong R, Rutka JT, Wang GL, Feng ZP, Sun HS. 2019; Role of Cl- channels in primary brain tumour. Cell Calcium. 81:1–11. DOI: 10.1016/j.ceca.2019.05.004. PMID: 31129471.
22. Jia L, Liu W, Guan L, Lu M, Wang K. 2015; Inhibition of calcium-activated chloride channel ANO1/TMEM16A suppresses tumor growth and invasion in human lung cancer. PLoS One. 10:e0136584. DOI: 10.1371/journal.pone.0136584. PMID: 26305547. PMCID: PMC4549304. PMID: 7be1e37575d04a47b36c71ff7d0abaff.

23. Lee HS, Kim Y. 2020; Aucklandia lappa causes cell wall damage in Candida albicans by reducing chitin and (1,3)-β-D-glucan. J Microbiol Biotechnol. 30:967–973. DOI: 10.4014/jmb.2002.02025. PMID: 32347080. PMCID: PMC9728168.

24. Wang W, Li Q, Yan X, Chen Z, Xie Y, Hu H, Wang Z. 2020; Comparative study of raw and processed Vladimiriae Radix on pharmacokinetic and anti-acute gastritis effect through anti-oxidation and anti-inflammation. Phytomedicine. 70:153224. DOI: 10.1016/j.phymed.2020.153224. PMID: 32353684.

25. Tastan P, Hajdú Z, Kúsz N, Zupkó I, Sinka I, Kivcak B, Hohmann J. 2019; Sesquiterpene lactones and flavonoids from Psephellus pyrrhoblepharus with antiproliferative activity on human gynecological cancer cell lines. Molecules. 24:3165. DOI: 10.3390/molecules24173165. PMID: 31480332. PMCID: PMC6749316. PMID: 509261a1ef5144a0843d3f582423d903.

26. Choi JY, Choi EH, Jung HW, Oh JS, Lee WH, Lee JG, Son JK, Kim Y, Lee SH. 2008; Melanogenesis inhibitory compounds from Saussureae Radix. Arch Pharm Res. 31:294–299. DOI: 10.1007/s12272-001-1154-0. PMID: 18409040.

27. Xu Z, Xu J, Sun S, Lin W, Li Y, Lu Q, Li F, Yang Z, Lu Y, Liu W. 2022; Mecheliolide elicits ROS-mediated ERS driven immunogenic cell death in hepatocellular carcinoma. Redox Biol. 54:102351. DOI: 10.1016/j.redox.2022.102351. PMID: 35671636. PMCID: PMC9168183.

Fig. 1
The chemical structures of Aucklandiae Radix compounds and MONNA investigated in this study.
The chemical structures of mecheliolide (A), costunolide (B), dehydrocostus lactone (C), and MONNA (D).
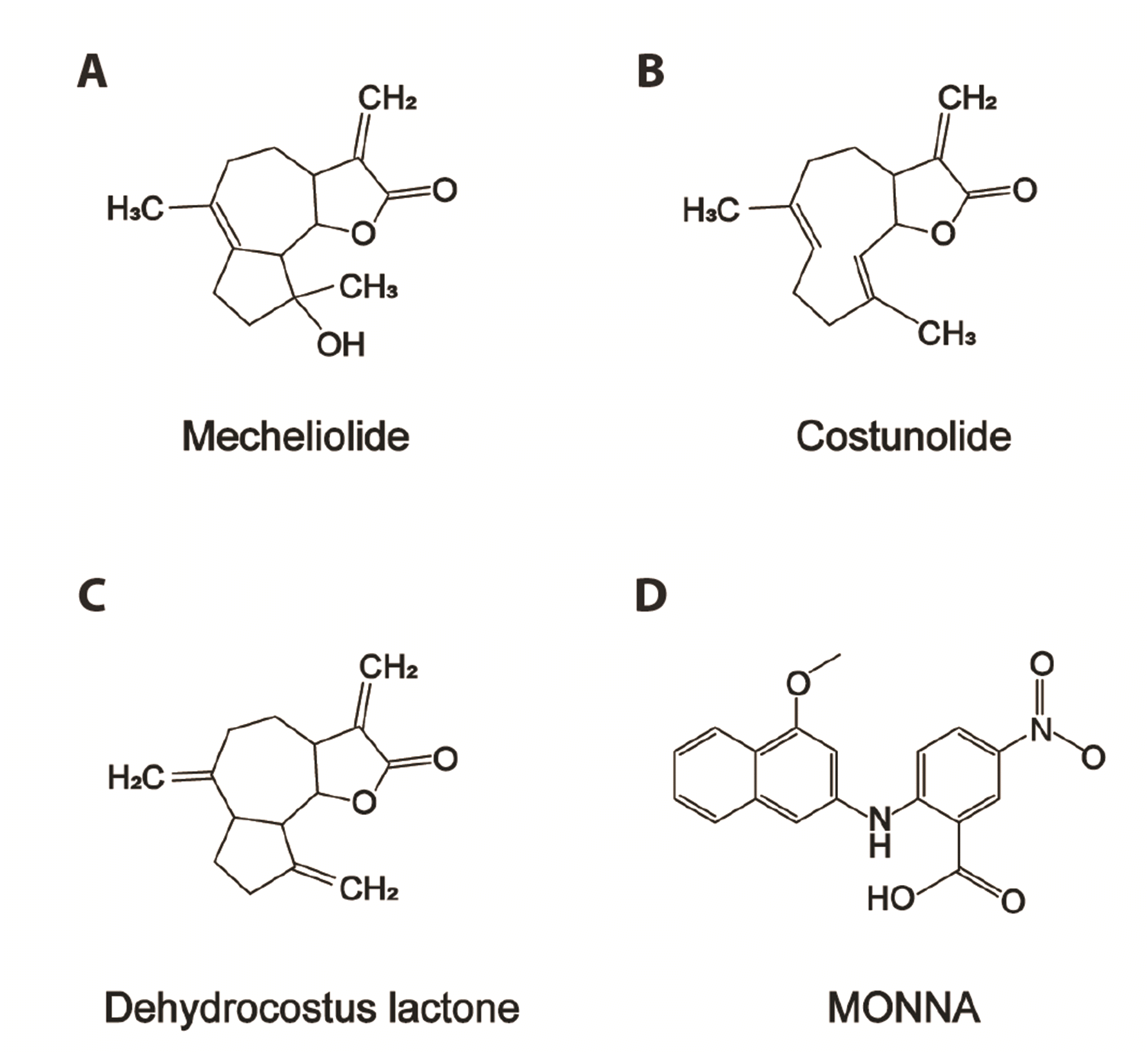
Fig. 2
The inhibitory effects of mecheliolide, costunolide and dehydrocostus lactone on recombinant TMEM16A-medicated CaCC currents in CHO cells.
Representative patch-clamp recordings of current traces at 300 nM Ca2+ in the presence of 100 μM mecheliolide (A), costunolide (B), dehydrocostus lactone (C), and 10 μM MONNA (D) in the bath medium. (E) Whole-cell TMEM16A-medicated CaCC currents were stimulated with a 4-s ramp voltage protocol from −80 mV to +80 mV. The holding potential was set to 0 mV. (F) Bar graph showing the inhibitory effects of mecheliolide (100 μM), costunolide (100 μM), dehydrocostus lactone (100 μM), and MONNA (10 μM) on TMEM16A-medicated CaCCs at +80 mV. Values are presented as mean ± SEM. Current traces recorded at the time points indicated by the letters (a, b). CaCC, Ca2+-activated Cl- channel; CHO, Chinese hamster ovary. *p < 0.05, vs. the current amplitudes in the absence of drugs; n = 5 cells, per group.
Fig. 3
The effects of mecheliolide, costunolide and dehydrocostus lactone on the I-V relationship of TMEM16A-mediated CaCC currents in CHO cells.
(A) Voltage step protocol. 1.5-s step pulses between –80 and +80 mV with increments of 20 mV from a holding potential of 0 mV followed by a –100 mV step. (B) Representative patch-clamp recordings of whole-cell TMEM16A-mediated CaCC currents evoked by 300 nM Ca2+ in the absence (control) or presence of 100 μM mecheliolide, costunolide and dehydrocostus lactone in the bath medium. The effects of 10 μM MONNA on the currents are shown in the right panels. (C–E) The mean normalized current-voltage relationships of whole-cell TMEM16A-mediated CaCC currents. n = 5 cells, per group. CaCC, Ca2+-activated Cl- channel; CHO, Chinese hamster ovary; I–V, current-voltage.
Fig. 4
The concentration-response relationships for mecheliolide, costunolide and dehydrocostus lactone on recombinant TMEM16A-mediated CaCC currents in CHO cells.
(A) The time course of the effects of mecheliolide (1, 3, 10, 30, 100 μM) on TMEM16A currents tested at +80 mV. (B) Representative TMEM16A-mediated CaCC current traces (recorded using the voltage protocol indicated in Fig. 2E) before and after the effects of different concentrations of mecheliolide were stabilized. (C) The concentration-response relationships of mecheliolide, costunolide and dehydrocostus lactone on TMEM16A-mediated CaCC currents measured at +80 mV. Data were fitted by a logistic function. (D) IC50 and Emax values of mecheliolide, costunolide and dehydrocostus lactone. n = 5 cells, per group. CaCC, Ca2+-activated Cl– channel; CHO, Chinese hamster ovary.
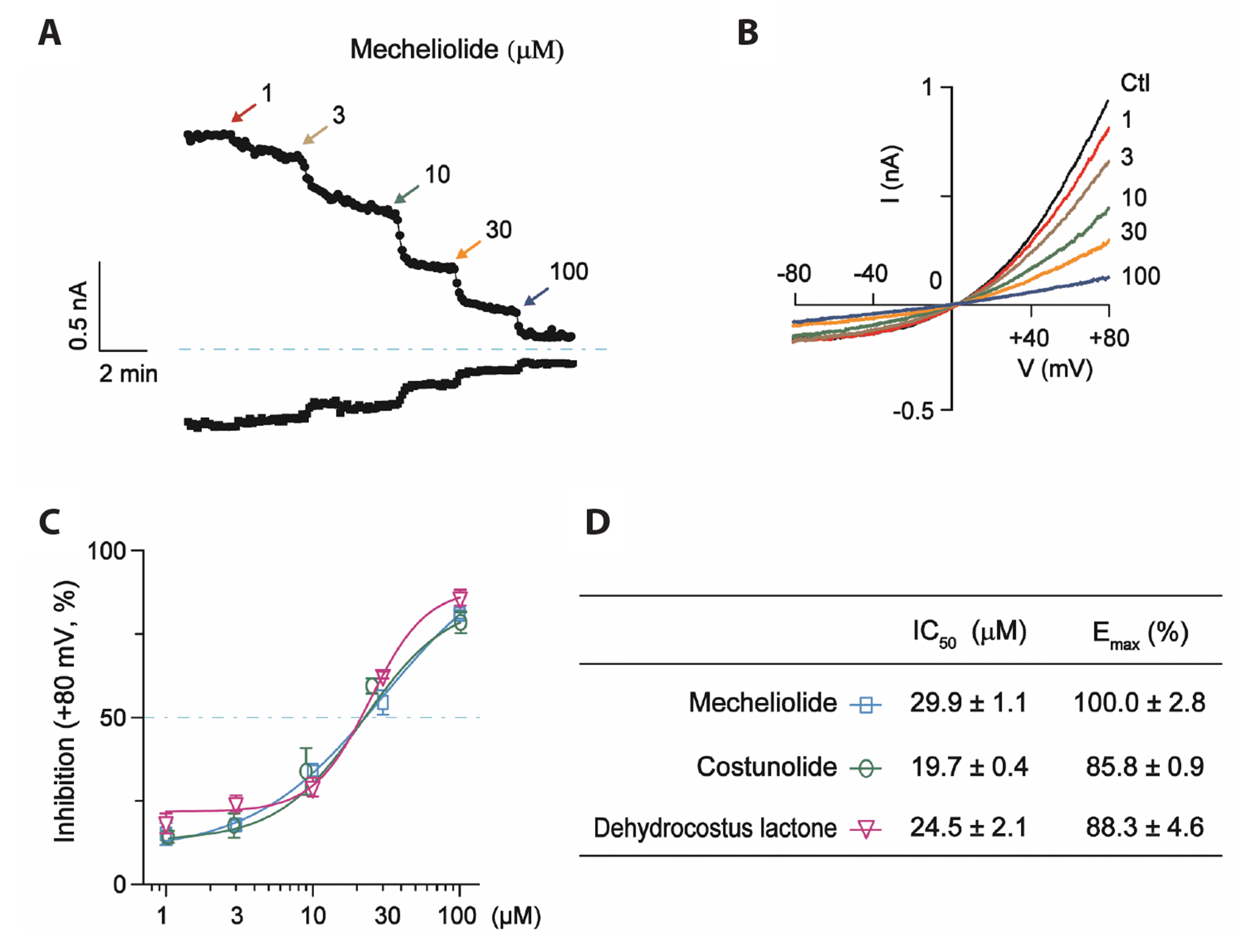
Fig. 5
The inhibitory effects of mecheliolide, costunolide and dehydrocostus lactone on endogenous TMEM16A-mediated CaCC currents in LA795 lung adenocarcinoma cells.
(A) The representative traces of endogenous TMEM16A-mediated CaCCs in LA795 cells recorded using voltage-command shown in Fig. 3A. Dotted lines indicate zero current amplitude. (B) Representative endogenous TMEM16A-mediated CaCC current traces in LA795 cells (recorded using the voltage protocol shown in Fig. 2E before and after the effects of 100 μM mecheliolide, costunolide and dehydrocostus lactone were stabilized. The effects of MONNA (10 μM) are also shown. (C) Bar graph showing the inhibitory effect of 100 μM mecheliolide, costunolide and dehydrocostus lactone and 10 μM MONNA on endogenous TMEM16A-mediated CaCCs. Values are presented as mean ± SEM. CaCC, Ca2+-activated Cl– channel. *p < 0.05, vs. the current amplitudes in the absence of drugs; n = 5 for each experimental group.
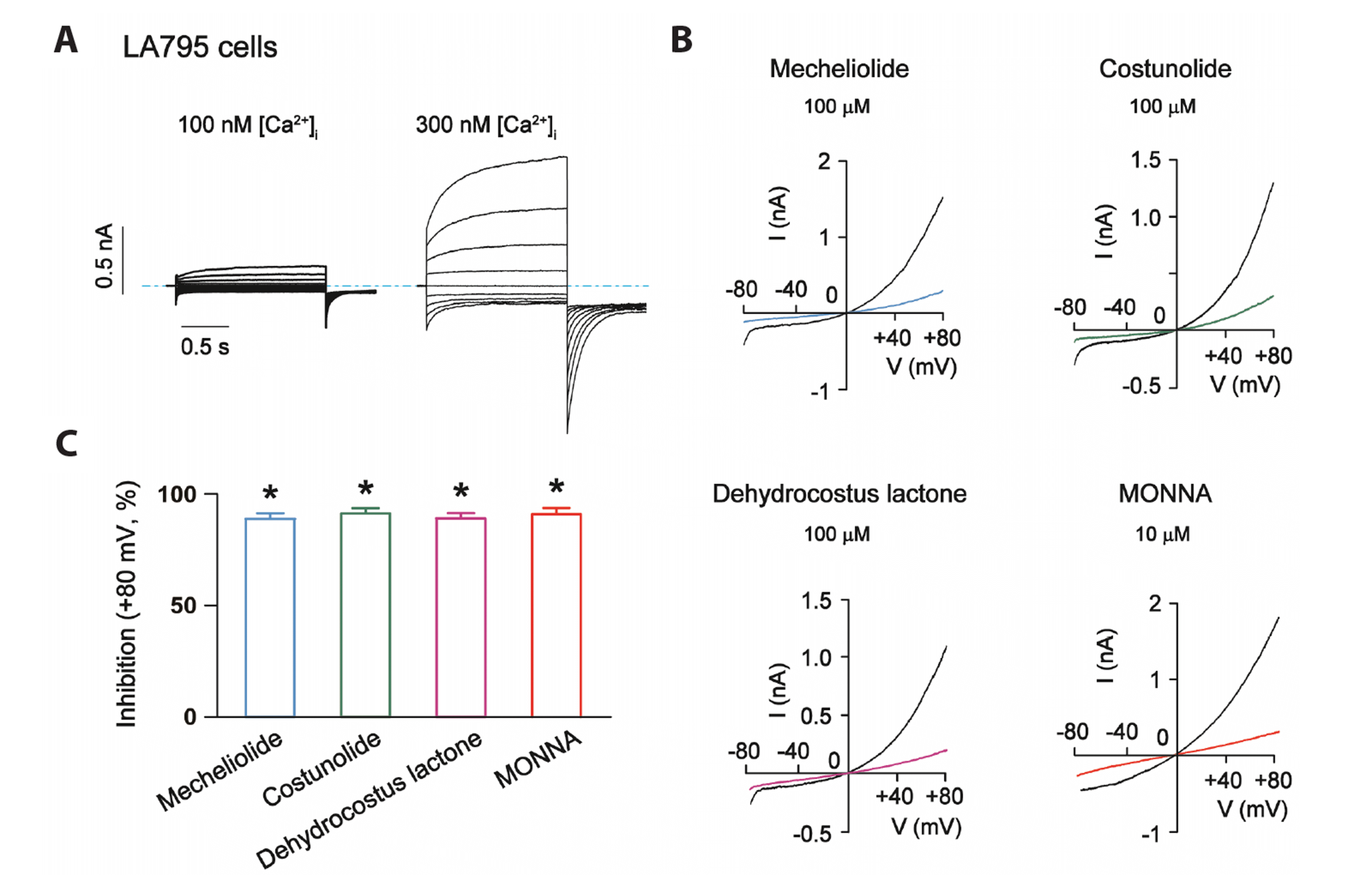
Fig. 6
The inhibitory effects of mecheliolide, costunolide and dehydrocostus lactone on the proliferation and migration of LA795 lung adenocarcinoma cells.
(A) Results of the CCK-8 assay. Representative images recorded after mecheliolide, costunolide and dehydrocostus lactone (1, 3, 10, 30, 100 μM) treatment for 24 h. (B–D) The concentration-response relationships for mecheliolide, costunolide and dehydrocostus lactone on cell proliferation. Data were fitted with the logistic function. (E, F) Representative images recorded after MONNA (1, 3, 10, 30, 100, 300 μM) treatment for 24 h. Bar graph show the quantification result. The results represent the means of three independent experiments. *p < 0.05, vs. the control cells in the absence of drugs.

Fig. 7
The inhibitory effects of mecheliolide, costunolide and dehydrocostus lactone on the migration of LA795 lung adenocarcinoma cells.
(A) Results of the Trans-well migration assay. Representative images recorded after mecheliolide, costunolide and dehydrocostus lactone (1, 3, 10, 30, 100 μM) treatment for 72 h. The concentration-response relationships for mecheliolide (B), costunolide (C) and dehydrocostus lactone (D) on cell migration. Data were fitted with the logistic function. (E, F) Representative images recorded after MONNA (1, 3, 10, 30, 100, 300 μM) treatment for 72 h. Bar graph show the quantification result. The results represent the means of three independent experiments. *p < 0.05, vs. the control cells in the absence of drugs.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download