Abstract
Cornuside is a secoiridoid glucoside compound extracted from the fruits of Cornus officinalis. Cornuside has immunomodulatory and anti-inflammatory properties; however, its potential therapeutic effects on diabetic nephropathy (DN) have not been completely explored. In this study, we established an in vitro model of DN through treating mesangial cells (MMCs) with glucose. MMCs were then treated with different concentrations of cornuside (0, 5, 10, and 30 μM). Cell viability was determined using cell counting kit-8 and 5-ethynyl-2′-deoxyuridine assays. Levels of pro-inflammatory cytokines, including interleukin (IL)-6, tumor necrosis factor-α, and IL-1β were examined using enzyme-linked immunosorbent assay. Reverse transcription quantitative real-time polymerase chain reaction and Western blotting were performed to detect the expression of AKT and nuclear factor-kappa B (NF-κB)-associated genes. We found that cornuside treatment significantly reduced glucose-induced increase in MMC viability and expression of pro-inflammatory cytokines. Moreover, cornuside inhibited glucose-induced phosphorylation of AKT and NF-κB inhibitor alpha, decreased the expression of proliferating cell nuclear antigen and cyclin D1, and increased the expression of p21. Our study indicates that the anti-inflammatory properties of cornuside in DN are due to AKT and NF-κB inactivation in MMCs.
Diabetic nephropathy (DN) is the primary cause of chronic kidney disease (CKD) and seriously affects the prognosis and survival of patients with diabetes [1,2]. The incidence of DN may involve hemodynamic and metabolic factors that significantly increase the levels of blood glucose and advanced glycation end products (AGEs), consequently leading to the activation of the renin–angiotensin–aldosterone system [3]. Currently, the management of diabetes focuses on reducing proteinuria through maintaining blood pressure and blood glucose levels; however, these interventions are unable to prevent the development of CKD [4]. Therefore, developing safe and effective methods to manage DN is necessary.
Results of previous studies have indicated that mesangial cells (MMCs) play a vital role in controlling mesangial matrix homeostasis, glomerular filtration rate, and normal glomerular function through regulating extracellular matrix (ECM) and producing cytokines. The progression of DN is characterized by overproliferation of MMCs, accumulation of ECM, thickening of the basement membrane, and expansion of the mesangial area, leading to the development of glomerulosclerosis [5]. Early glomerular mesangial lesions, characterized by MMC proliferation, are among the most prominent pathological changes in DN [6].
Inflammation is stimulated in response to detrimental conditions to maintain tissue homeostasis and integrity [7]. Long-term chronic inflammation can lead to the progression of different diseases [8]. Accumulating evidence suggests that inflammation is involved in the progression of DN [9]. As reported previously, DN was accompanied with an increased production of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 [10]. Hence, inhibition of inflammation has become a potential strategy for the treatment of DN.
In recent years, various traditional Chinese medicines have been proposed to participate in multiple cellular activities such as cell proliferation, apoptosis, and differentiation [11]. Cornuside is a secoiridoid glucoside extracted from the fruit of Cornus officinalis, a traditional oriental medicine [12]. Crude extracts of C. officinalis have been shown to exert antineoplastic, anti-inflammatory, hepatoprotective, and anti-sepsis effects [13]. Of note, the results of a previous study revealed that loganin and its derivatives are the active compounds in the C. officinalis fruit and may exert anti-DN functions [14]. However, the role of cornusides in DN remains unclear.
In this study, we established an in vitro model of DN through treating MMCs with glucose and aimed to explore the possible underlying mechanism of the antidiabetic effects of cornuside.
Mice MMC line SV40-MES13 was purchased from Procell and cultured in DMEM/F12 medium (Thermo Fisher Scientific) containing 10% fetal bovine serum and 1% penicillin–streptomycin at 37°C with 5% CO2. Cells were digested with trypsin for passaging when they reached approximately 90% confluence.
MMCs in the logarithmic phase were treated with glucose (0, 10, 25, 50, and 100 mM) for 24 h to establish an in vitro DN cell model. Cells were cultured at 37°C with 5% CO2 for 24 h, followed by the subsequent analyses.
MMCs (5 × 103 cells/well) were cultured for 24 h under the different treatments. CCK-8 reagents (10 μl; Beyotime) were added into each well and maintained for 2 h. The absorbance was measured at 450 nm using a microplate reader (Bio-Rad).
MMCs were cultured in 96-well plates and treated with 50 µM of EdU-labeling medium for 2 h. After treatment with 4% paraformaldehyde and 0.5% Triton X-100, the cells were stained with an anti-EdU working solution and photographed using a fluorescent microscope (Olympus).
The expression levels of IL-6, IL-1β, and TNF-α were examined using commercially available assay kits (Thermo Fisher Scientific). Briefly, cell culture supernatants were collected and added to a 96-well plate coated with IL-6, IL-1β, and TNF-α antibodies for incubation and then washed with PBS containing 0.1% Tween 20. After reacting with primary and secondary antibodies, a chemiluminescent substrate was added and incubated at 37°C for 15–30 min. The optical density was measured using a microplate reader (Bio-tek) at 450 nm after the addition of the stop solution.
Trizol reagent (Invitrogen) was used to extract total RNAs. The RNA samples were then reverse transcribed into cDNA, followed by quantitative PCR using a commercially available kit (One Step TB Green PrimeScript RT-PCR Kit [Perfect Real Time]; Takara). The sequences of the primers were: p21: F 5ʹ-TAAGGACGTCCCACTTTGCC-3ʹ, R 5ʹ-CGTCT CCGTGACGAAGTCAA-3ʹ; proliferating cell nuclear antigen (PCNA): F 5ʹ-GAACCTCACCAGCATGTCCA-3ʹ, R 5ʹ-ATTCACCCGACGGCATCTTT-3ʹ; cyclin D1: F 5ʹ-AAAATGCCAGAGGCGGATGA-3ʹ, R 5ʹ-GAGGGG GTCCTTGTTTAGCC-3ʹ; AKT: F 5ʹ-CCGCCTGATCAA GTTCTCCT-3ʹ, R 5ʹ-GCCACATGTGTGGTCTCAAAC-3ʹ; GAPDH: F 5ʹ-CCCTTA AGAGGGATGCTGCC-3ʹ, R 5ʹ-TTCCCATTCTCGGCCTTGAC-3ʹ.
MMCs were lysed using RIPA lysates (Beyotime Biotechnology) and then separated by 10% SDS-PAGE, followed by transfer to PVDF membranes (Millipore). The membranes were sealed and then incubated overnight with the following primary antibodies, including PCNA (ab18197, Abcam), cyclin D1 (ab16663, Abcam), p21 (ab109520, Abcam), p65 (ab32536, Abcam), AKT (ab283852, Abcam), p-AKT (ab38449, Abcam), p-IκB-α (FS-K0390, affandi-e), COL1 (ab275746, Abcam), fibronectin (ab2413, Abcam), lamin A/C (ab108595, Abcam), and β-actin (ab8226, Abcam). Next, the membranes were washed with corresponding secondary antibodies and visualized using an ECL detection kit (Millipore).
Fig. 1A shows the chemical structure of cornusides. Next, MMCs were treated with glucose (0, 10, 25, 50, and 100 mM) for 12, 24, and 48 h to establish an in vitro model of DN. We then assessed cell viability upon glucose treatment. We found that 25, 50, and 100 mM markedly increased the viability of MMCs in a time-dependent manner (Fig. 1B); however, the effects of 25, 50, and 100 mM were not significant at each time point. Therefore, 25 mM glucose was used in subsequent experiments.
Next, to evaluate the impacts of cornuside on the growth of glucose-treated MMCs, 25 mM of treated MMCs were co-treated with cornuside (5, 10 or 30 μM), and the viability as well as proliferation of the MMCs were determined using CCK-8 and EdU assays. We found that 25 mM glucose markedly increased the viability and proliferation of MMCs, whereas cornuside inhibited the viability (Fig. 2A) and proliferation (Fig. 2B) of glucose-treated MMCs in a dose-dependent manner.
We further evaluated the influence of cornuside on the expression of proinflammatory cytokines in high glucose-treated MMCs. As revealed in Fig. 3, high glucose stimulation significantly increased the expressions of IL-6, TNF-α, and IL-1β in contrast to the control group. However, cornuside significantly decreased the expression of IL-6, TNF-α, and IL-1β in glucose-treated MMCs in a dose-dependent manner.
We further examined the influence of cornuside on the activation of AKT and NF-κB signaling pathways in high glucose-treated-induced MMCs. Through RT-qPCR analysis, we observed that high glucose treatment significantly increased the mRNA expression of PCNA and cyclin D1 and suppressed p21 mRNA expression. Concurrently, co-treatment with cornuside markedly decreased the mRNA levels of PCNA and cyclin D1 and increased the mRNA level of p21 in a dose-dependent manner. Cornuside did not affect AKT mRNA expression (Fig. 4A). Moreover, high glucose increased the protein expression of PCNA, cyclin D1, p-AKT p-IκB-α, COL1, and fibronectin, while reducing protein expression of p21. Conversely, cornuside treatment decreased the protein expression of PCNA, cyclin D1, p-AKT, p-IκB-α, COL1, and fibronectin but enhanced protein expression of p21 in a dose-dependent manner (Fig. 4B). Additionally, results of western blot analysis also reflected the translocation of p65 into the nucleus upon high glucose induction (Fig. 4C). Concurrently, cornuside treatment obviously repressed the translocation of p65 in a dose-dependent manner.
Overproliferation of glomerular MMCs and inflammatory conditions are prominent pathological characteristics of DN [15]. High glucose can activate inflammatory pathways, e.g., NF-κB signaling pathway along with the increased production of pro-inflammatory cytokines [16]. C. officinalis is a dietary anti-inflammatory agent widely used clinically [17]. Previous studies have suggested that the fruit of C. officinalis can be used to treat backache, hypertension, and polyuria [18]. In addition, a previous study revealed the biological properties of C. officinalis, including anti-inflammatory, renal, and hepatic protective roles, and immunomodulation [19]. Studies have shown that Cornuside I can improve osteoporosis through inducing bone mesenchymal stem cell proliferation [20]. In this study, we established an in vitro model of DN, and the results of CCK-8 and EdU analyses showed markedly reduced glucose-induced proliferation of MMCs. These results suggest the anti-proliferative functions of cornuside.
Conversely, the immunomodulatory and anti-inflammatory activities of cornuside in various diseases have been discussed previously [21]. Kang et al. [22] reported that cornuside inhibited cytokine-induced pro-inflammatory effects in human umbilical vein endothelial cells. Zhang et al. [23] found that cornuside suppressed experimental autoimmune encephalomyelitis in the central nervous system. Moreover, Choi et al. [24] suggested that cornuside suppresses lipopolysaccharide-induced increases in inflammatory mediator production. In this study, we found that cornuside could alleviate high glucose-induced production of pro-inflammatory cytokines, i.e., IL-6, TNF-α, and IL-1β, which further confirmed the anti-inflammatory functions of cornuside.
NF-κB signaling is crucial during the development of DN [25]. NF-κB inhibitor alpha (IκB-α) is an important inflammation mediator, which modulates the activation of p50/p65 heterodimer, and then the p50/p65 subunits consequentially translocate into the nucleus and bind with the target inflammatory genes upon IκB-α degradation [26]. In addition, the IκB-α/NF-κB is actively involved in the progression of DN [27]. In this study, we found that cornuside reduced the translocation of activated NF-κB to the nucleus and also inhibited the phosphorylation of IκB-α suggesting that cornuside suppressed the IκB-α/NF-κB signaling pathway during DN progression.
Recent studies have shown that inflammation and ECM accumulation play important roles in the progression of DN. Chronic hyperglycemia may increase the levels of AGEs [28], while AGEs increase the production of transforming growth factor-1 (TGF-β1) in glomerular cells, which can lead to abnormal production of ECMs, inducing glomerular sclerosis and interstitial tubule damage [29]. Therefore, the inhibition of ECM protein production may be an effective method for the prevention and treatment of DN. Our results showed that cornuside inhibited HG-induced overexpression of COL1 and fibronectin. Furthermore, previous studies have shown that an increase in AGEs can stimulate NF-κB signaling, leading to an increase in ECM synthesis [30]. Our results suggest that cornuside may relieve DN by inhibiting NF-κB signaling, which, in turn, decreases ECM synthesis and improves DN symptoms.
PI3K/AKT signaling plays an important role in inflammation-mediated diseases, including DN [31-33]. For instance, microRNA-126 may inhibit inflammation and apoptosis in DN via regulating the PI3K/AKT signaling pathway [34]. Furthermore, tetramethylpyrazine alleviates DN through activating AKT signaling [35]. Herein, we evaluated the effects of cornuside on the expression of key molecules involved in the AKT signaling pathway. We found that cornuside decreased the expression of PCNA, cyclin D1, and p-AKT and increased the expression of p21, implying that cornuside inhibited the AKT signaling pathway during the progression of DN.
In summary, the results of this study indicate that cornuside can inhibit the proliferation of high glucose-treated MMCs and alleviate the inflammatory condition through suppressing the AKT and NF-κB signaling pathways. Therefore, the results of this study demonstrate that cornuside can serve as an alternative medication for the treatment of DN.
REFERENCES
1. Valk EJ, Bruijn JA, Bajema IM. 2011; Diabetic nephropathy in humans: pathologic diversity. Curr Opin Nephrol Hypertens. 20:285–289. DOI: 10.1097/MNH.0b013e328345bc1c. PMID: 21422920.

2. Umanath K, Lewis JB. 2018; Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis. 71:884–895. DOI: 10.1053/j.ajkd.2017.10.026. PMID: 29398179.

3. Anders HJ, Huber TB, Isermann B, Schiffer M. 2018; CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 14:361–377. DOI: 10.1038/s41581-018-0001-y. PMID: 29654297.

4. Kosmas CE, Silverio D, Sourlas A, Garcia F, Montan PD, Guzman E. 2018; Impact of lipid-lowering therapy on glycemic control and the risk for new-onset diabetes mellitus. Drugs Context. 7:212562. DOI: 10.7573/dic.212562. PMID: 30515229. PMCID: PMC6267678. PMID: 0359c64300c7488ea0c4f4832ef66955.

5. A/L B Vasanth Rao VR, Tan SH, Candasamy M, Bhattamisra SK. 2019; Diabetic nephropathy: an update on pathogenesis and drug development. Diabetes Metab Syndr. 13:754–762. DOI: 10.1016/j.dsx.2018.11.054. PMID: 30641802.

6. Gao P, Li L, Yang L, Gui D, Zhang J, Han J, Wang J, Wang N, Lu J, Chen S, Hou L, Sun H, Xie L, Zhou J, Peng C, Lu Y, Peng X, Wang C, Miao J, Ozcan U, et al. 2019; Yin Yang 1 protein ameliorates diabetic nephropathy pathology through transcriptional repression of TGFβ1. Sci Transl Med. 11:eaaw2050. DOI: 10.1126/scitranslmed.aaw2050. PMID: 31534017.

7. Hotamisligil GS. 2006; Inflammation and metabolic disorders. Nature. 444:860–867. DOI: 10.1038/nature05485. PMID: 17167474.

8. Medzhitov R. 2008; Origin and physiological roles of inflammation. Nature. 454:428–435. DOI: 10.1038/nature07201. PMID: 18650913.

9. Tuttle KR. 2005; Linking metabolism and immunology: diabetic nephropathy is an inflammatory disease. J Am Soc Nephrol. 16:1537–1538. DOI: 10.1681/ASN.2005040393. PMID: 15872083.

10. Li F, Chen Y, Li Y, Huang M, Zhao W. 2020; Geniposide alleviates diabetic nephropathy of mice through AMPK/SIRT1/NF-κB pathway. Eur J Pharmacol. 886:173449. DOI: 10.1016/j.ejphar.2020.173449. PMID: 32758570.

11. Su Y, Qin W, Wu L, Yang B, Wang Q, Kuang H, Cheng G. 2021; A review of Chinese medicine for the treatment of psoriasis: principles, methods and analysis. Chin Med. 16:138. DOI: 10.1186/s13020-021-00550-y. PMID: 34930402. PMCID: PMC8686297. PMID: 817e6e8dce43484ca4a103f6f54af30f.

12. Ryu SH, Kim C, Kim N, Lee W, Bae JS. 2022; Inhibitory functions of cornuside on TGFBIp-mediated septic responses. J Nat Med. 76:451–461. DOI: 10.1007/s11418-021-01601-2. PMID: 35025027. PMCID: PMC8757402.

13. Czerwińska ME, Melzig MF. 2018; Cornus mas and Cornus Officinalis-analogies and differences of two medicinal plants traditionally used. Front Pharmacol. 9:894. DOI: 10.3389/fphar.2018.00894. PMID: 30210335. PMCID: PMC6121078. PMID: c372e0d5a3d44654a1df8074afeb019e.
14. Ma W, Wang KJ, Cheng CS, Yan GQ, Lu WL, Ge JF, Cheng YX, Li N. 2014; Bioactive compounds from Cornus officinalis fruits and their effects on diabetic nephropathy. J Ethnopharmacol. 153:840–845. DOI: 10.1016/j.jep.2014.03.051. PMID: 24694395.

15. Wang Q. 2022; XIST silencing alleviated inflammation and mesangial cells proliferation in diabetic nephropathy by sponging miR-485. Arch Physiol Biochem. 128:1697–1703. DOI: 10.1080/13813455.2020.1789880. PMID: 32669002.

16. Saengboonmee C, Phoomak C, Supabphol S, Covington KR, Hampton O, Wongkham C, Gibbs RA, Umezawa K, Seubwai W, Gingras MC, Wongkham S. 2020; NF-κB and STAT3 co-operation enhances high glucose induced aggressiveness of cholangiocarcinoma cells. Life Sci. 262:118548. DOI: 10.1016/j.lfs.2020.118548. PMID: 33038372. PMCID: PMC7686287.

17. Gao X, Liu Y, An Z, Ni J. 2021; Active components and pharmacological effects of Cornus officinalis: literature review. Front Pharmacol. 12:633447. DOI: 10.3389/fphar.2021.633447. PMID: 33912050. PMCID: PMC8072387. PMID: ed28052d7152464b8a995551b4bf352d.

18. Quah Y, Lee SJ, Lee EB, Birhanu BT, Ali MS, Abbas MA, Boby N, Im ZE, Park SC. 2020; Cornus officinalis ethanolic extract with potential anti-allergic, anti-inflammatory, and antioxidant activities. Nutrients. 12:3317. DOI: 10.3390/nu12113317. PMID: 33138027. PMCID: PMC7692184. PMID: c72cd6fbd95b4edf80cdbcdaede9da3c.

19. Fernando PDSM, Piao MJ, Zhen AX, Ahn MJ, Yi JM, Choi YH, Hyun JW. 2020; Extract of Cornus officinalis protects keratinocytes from particulate matter-induced oxidative stress. Int J Med Sci. 17:63–70. DOI: 10.7150/ijms.36476. PMID: 31929739. PMCID: PMC6945560.

20. Gao F, Xia SL, Wang XH, Zhou XX, Wang J. 2021; Cornuside I promoted osteogenic differentiation of bone mesenchymal stem cells through PI3K/Akt signaling pathway. J Orthop Surg Res. 16:397. DOI: 10.1186/s13018-021-02508-0. PMID: 34154621. PMCID: PMC8218506. PMID: 6079757930e44346b3144cfd44c97e99.

21. Li L, Jin G, Jiang J, Zheng M, Jin Y, Lin Z, Li G, Choi Y, Yan G. 2016; Cornuside inhibits mast cell-mediated allergic response by down-regulating MAPK and NF-κB signaling pathways. Biochem Biophys Res Commun. 473:408–414. DOI: 10.1016/j.bbrc.2016.03.007. PMID: 26972254.

22. Kang DG, Moon MK, Lee AS, Kwon TO, Kim JS, Lee HS. 2007; Cornuside suppresses cytokine-induced proinflammatory and adhesion molecules in the human umbilical vein endothelial cells. Biol Pharm Bull. 30:1796–1799. DOI: 10.1248/bpb.30.1796. PMID: 17827743.

23. Zhang R, Liu J, Xu B, Wu Y, Liang S, Yuan Q. 2021; Cornuside alleviates experimental autoimmune encephalomyelitis by inhibiting Th17 cell infiltration into the central nervous system. J Zhejiang Univ Sci B. 22:421–430. DOI: 10.1631/jzus.B2000771. PMID: 33973423. PMCID: PMC8110462.

24. Choi YH, Jin GY, Li GZ, Yan GH. 2011; Cornuside suppresses lipopolysaccharide-induced inflammatory mediators by inhibiting nuclear factor-kappa B activation in RAW 264.7 macrophages. Biol Pharm Bull. 34:959–966. DOI: 10.1248/bpb.34.959. PMID: 21719998.

25. Wada J, Makino H. 2013; Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond). 124:139–152. DOI: 10.1042/CS20120198. PMID: 23075333.

26. Yu H, Lin L, Zhang Z, Zhang H, Hu H. 2020; Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. 5:209. DOI: 10.1038/s41392-020-00312-6. PMID: 32958760. PMCID: PMC7506548.

27. Yi H, Peng R, Zhang LY, Sun Y, Peng HM, Liu HD, Yu LJ, Li AL, Zhang YJ, Jiang WH, Zhang Z. 2017; LincRNA-Gm4419 knockdown ameliorates NF-κB/NLRP3 inflammasome-mediated inflammation in diabetic nephropathy. Cell Death Dis. 8:e2583. DOI: 10.1038/cddis.2016.451. PMID: 28151474. PMCID: PMC5386454.

28. Wu L, Liu C, Chang DY, Zhan R, Sun J, Cui SH, Eddy S, Nair V, Tanner E, Brosius FC, Looker HC, Nelson RG, Kretzler M, Wang JC, Xu M, Ju W, Zhao MH, Chen M, Zheng L. 2021; Annexin A1 alleviates kidney injury by promoting the resolution of inflammation in diabetic nephropathy. Kidney Int. 100:107–121. Erratum in: Kidney Int. 2021;100:1349-1350. DOI: 10.1016/j.kint.2021.02.025. PMID: 33675846. PMCID: PMC8893600.

29. Zhao Y, Zhang W, Jia Q, Feng Z, Guo J, Han X, Liu Y, Shang H, Wang Y, Liu WJ. 2018; High dose vitamin E attenuates diabetic nephropathy via alleviation of autophagic stress. Front Physiol. 9:1939. DOI: 10.3389/fphys.2018.01939. PMID: 30719008. PMCID: PMC6348272. PMID: d7fb718cd2e14e8297b2897e2877bf73.

30. Sun Z, Ma Y, Chen F, Wang S, Chen B, Shi J. 2018; Artesunate ameliorates high glucose-induced rat glomerular mesangial cell injury by suppressing the TLR4/NF-κB/NLRP3 inflammasome pathway. Chem Biol Interact. 293:11–19. DOI: 10.1016/j.cbi.2018.07.011. PMID: 30031708.

31. Sun X, Chen L, He Z. 2019; PI3K/Akt-Nrf2 and anti-inflammation effect of macrolides in chronic obstructive pulmonary disease. Curr Drug Metab. 20:301–304. DOI: 10.2174/1389200220666190227224748. PMID: 30827233.

32. Xu Z, Jia K, Wang H, Gao F, Zhao S, Li F, Hao J. 2021; METTL14-regulated PI3K/Akt signaling pathway via PTEN affects HDAC5-mediated epithelial-mesenchymal transition of renal tubular cells in diabetic kidney disease. Cell Death Dis. 12:32. DOI: 10.1038/s41419-020-03312-0. PMID: 33414476. PMCID: PMC7791055. PMID: 958ea652b9b94c8e833522db485802c6.

33. Hou B, Li Y, Li X, Zhang C, Zhao Z, Chen Q, Zhang N, Li H. 2020; HGF protected against diabetic nephropathy via autophagy-lysosome pathway in podocyte by modulating PI3K/Akt-GSK3β-TFEB axis. Cell Signal. 75:109744. DOI: 10.1016/j.cellsig.2020.109744. PMID: 32827692.

34. Lou Z, Li Q, Wang C, Li Y. 2022; The effects of microRNA-126 reduced inflammation and apoptosis of diabetic nephropathy through PI3K/AKT signalling pathway by VEGF. Arch Physiol Biochem. 128:1265–1274. DOI: 10.1080/13813455.2020.1767146. PMID: 32449863.

35. Rai U, Kosuru R, Prakash S, Tiwari V, Singh S. 2019; Tetramethylpyrazine alleviates diabetic nephropathy through the activation of Akt signalling pathway in rats. Eur J Pharmacol. 865:172763. DOI: 10.1016/j.ejphar.2019.172763. PMID: 31682792.

Fig. 1
Glucose treatment increased viability of MMCs.
(A) The chemical structure of cornuside. (B) CCK-8 assay detected the cell viability after different doses of glucose treatment (0, 10, 25, 50, and 100 mM). Values are presented as mean ± SD. MMCs, mesangial cells; CCK-8, cell counting kit-8; OD, optical density. *p < 0.05, **p < 0.01.
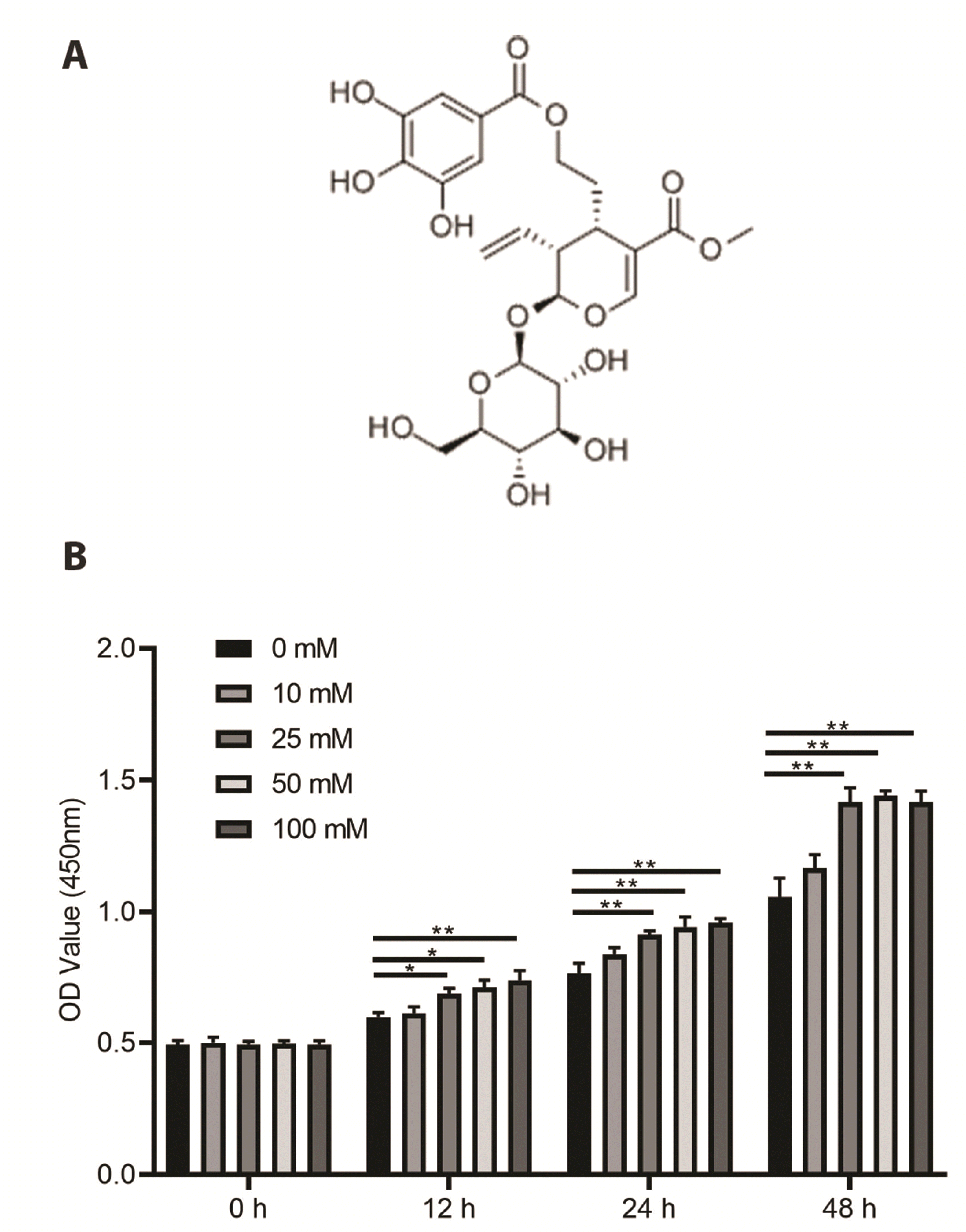
Fig. 2
Cornuside reduced high glucose-induced over-proliferation of MMCs.
CCK-8 (A) and EdU (B) assays assessed the viability and proliferation in high glucose-induced MMCs after different doses of cornuside treatment (0, 5, 10, and 30 μM). Values are presented as mean ± SD. MMCs, mesangial cells; CCK-8, cell counting kit-8; EdU, 5-ethynyl-2′-deoxyuridine; OD, optical density. *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 3
Cornuside inhibited the production of IL-6, TNF-α and IL-1β in high glucose-treated MMCs.
ELISA was conducted to determine the secretions of IL-6, TNF-α and IL-1β in high glucose-induced MMCs after different doses of cornuside treatment (0, 5, 10, and 30 μM). Values are presented as mean ± SD. MMCs, mesangial cells; IL, interleukin; TNF-α, tumor necrosis factor-α. *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 4
Cornuside inhibited the AKT and NF-κB signaling pathways in high glucose-treated MMCs.
(A) RT-qPCR analysis measured the mRNA levels of PCNA, cyclin D1, p21 and AKT in high glucose-treated MMCs after different doses of cornuside treatment (0, 5, 10, and 30 μM). (B) Western blot analysis measured the protein levels of p65, PCNA, cyclin D1, p21, AKT, p-AKT, p-IκB-α, COL1 and fibronectin in high glucose-induced MMCs after different doses of cornuside treatment (0, 5, 10, and 30 μM). (C) Western blot analysis measured the protein levels of p65 (nuclear) and p65 (cytosol) in high glucose-treated MMCs after different doses of cornuside treatment (0, 5, 10, and 30 μM). Values are presented as mean ± SD. MMCs, mesangial cells; NF-κB, nuclear factor-kappa B; RT-qPCR, reverse transcription quantitative real-time polymerase chain reaction; PCNA, proliferating cell nuclear antigen. *p < 0.05, **p < 0.01, ***p < 0.001.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download