1. European Association for the Study of the Liver. European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56(4):908–943. PMID:
22424438.
2. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022; 76(3):681–693. PMID:
34801630.
3. Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010; 52(2):762–773. PMID:
20564355.
4. Lee MW, Kim YJ, Park HS, Yu NC, Jung SI, Ko SY, et al. Targeted sonography for small hepatocellular carcinoma discovered by CT or MRI: factors affecting sonographic detection. AJR Am J Roentgenol. 2010; 194(5):W396–W400. PMID:
20410384.
5. Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol. 2006; 59(3):432–441. PMID:
16690240.
6. Zhang Y, Zhang MW, Fan XX, Mao DF, Ding QH, Zhuang LH, et al. Drug-eluting beads transarterial chemoembolization sequentially combined with radiofrequency ablation in the treatment of untreated and recurrent hepatocellular carcinoma. World J Gastrointest Surg. 2020; 12(8):355–368. PMID:
32903981.
7. Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006; 12(8):2563–2567. PMID:
16638866.
8. Lee KH, Liapi EA, Cornell C, Reb P, Buijs M, Vossen JA, et al. Doxorubicin-loaded QuadraSphere microspheres: plasma pharmacokinetics and intratumoral drug concentration in an animal model of liver cancer. Cardiovasc Intervent Radiol. 2010; 33(3):576–582. PMID:
20087738.
9. Lee KH, Joo SM, Yum TJ, Jung SH. Conventional versus drug-eluting beads trans-arterial chemoembolization for treatment of hepatocellular carcinoma at very early and early stages. J Liver Cancer. 2017; 17(2):144–152.
10. Kim JW, Kim JH, Sung KB, Ko HK, Shin JH, Kim PN, et al. Transarterial chemoembolization vs. radiofrequency ablation for the treatment of single hepatocellular carcinoma 2 cm or smaller. Am J Gastroenterol. 2014; 109(8):1234–1240. PMID:
24935276.
11. Suh YJ, Jin YJ, Jeong Y, Shin WY, Lee JM, Cho S, et al. Resection or ablation versus transarterial therapy for Child-Pugh A patients with a single small hepatocellular carcinoma. Medicine (Baltimore). 2021; 100(43):e27470. PMID:
34713824.
12. Hsu CY, Huang YH, Chiou YY, Su CW, Lin HC, Lee RC, et al. Comparison of radiofrequency ablation and transarterial chemoembolization for hepatocellular carcinoma within the Milan criteria: a propensity score analysis. Liver Transpl. 2011; 17(5):556–566. PMID:
21506244.
13. Kim TH, Kim NH, Kim JD, Kim YN, Kim YJ, Kim EJ, et al. Transarterial chemoembolization using drug-eluting bead compared with radiofrequency ablation for treatment of single small hepatocellular carcinoma: a pilot non-randomized trial. J Liver Cancer. 2021; 21(2):146–154. PMID:
37383084.
14. Korean Liver Cancer Study Group (KLCSG). National Cancer Center, Korea (NCC). 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015; 16(3):465–522. PMID:
25995680.
15. Gaba RC, Lewandowski RJ, Hickey R, Baerlocher MO, Cohen EI, Dariushnia SR, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2016; 27(4):457–473. PMID:
26851158.
16. Goldberg SN, Charboneau JW, Dodd GD 3rd, Dupuy DE, Gervais DA, Gillams AR, et al. Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology. 2003; 228(2):335–345. PMID:
12893895.
17. Punt CJ, Buyse M, Köhne CH, Hohenberger P, Labianca R, Schmoll HJ, et al. Endpoints in adjuvant treatment trials: a systematic review of the literature in colon cancer and proposed definitions for future trials. J Natl Cancer Inst. 2007; 99(13):998–1003. PMID:
17596575.
18. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005; 235(3):728–739. PMID:
15845798.
19. Lam VW, Ng KK, Chok KS, Cheung TT, Yuen J, Tung H, et al. Incomplete ablation after radiofrequency ablation of hepatocellular carcinoma: analysis of risk factors and prognostic factors. Ann Surg Oncol. 2008; 15(3):782–790. PMID:
18095030.
20. Kang TW, Lim HK, Lee MW, Kim YS, Choi D, Rhim H. Perivascular versus nonperivascular small HCC treated with percutaneous RF ablation: retrospective comparison of long-term therapeutic outcomes. Radiology. 2014; 270(3):888–899. PMID:
24475820.
21. Peng ZW, Lin XJ, Zhang YJ, Liang HH, Guo RP, Shi M, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012; 262(3):1022–1033. PMID:
22357902.
22. Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007; 188(2):480–488. PMID:
17242258.
23. Lee M, Chung JW, Lee KH, Won JY, Chun HJ, Lee HC, et al. Korean multicenter registry of transcatheter arterial chemoembolization with drug-eluting embolic agents for nodular hepatocellular carcinomas: six-month outcome analysis. J Vasc Interv Radiol. 2017; 28(4):502–512. PMID:
27856136.
24. Idée JM, Guiu B. Use of Lipiodol as a drug-delivery system for transcatheter arterial chemoembolization of hepatocellular carcinoma: a review. Crit Rev Oncol Hematol. 2013; 88(3):530–549. PMID:
23921081.
25. Lee DH, Lee JM, Lee JY, Kim SH, Yoon JH, Kim YJ, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014; 270(3):900–909. PMID:
24475823.
26. Woo HY, Heo J. Transarterial chemoembolization using drug eluting beads for the treatment of hepatocellular carcinoma: now and future. Clin Mol Hepatol. 2015; 21(4):344–348. PMID:
26770921.
27. Facciorusso A, Mariani L, Sposito C, Spreafico C, Bongini M, Morosi C, et al. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2016; 31(3):645–653. PMID:
26331807.
28. Malagari K, Pomoni M, Kelekis A, Pomoni A, Dourakis S, Spyridopoulos T, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010; 33(3):541–551. PMID:
19937027.
29. Razi M, Safiullah S, Gu J, He X, Razi M, Kong J. Comparison of tumor response following conventional versus drug-eluting bead transarterial chemoembolization in early- and very early-stage hepatocellular carcinoma. J Interv Med. 2021; 5(1):10–14. PMID:
35586278.
30. Zou JH, Zhang L, Ren ZG, Ye SL. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: a meta-analysis. J Dig Dis. 2016; 17(8):510–517. PMID:
27384075.
31. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006; 44(1):217–231. PMID:
16298014.
32. Fleck A, Raines G, Hawker F, Trotter J, Wallace PI, Ledingham IM, et al. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. 1985; 325(8432):781–784.
33. Wang H, Cao C, Wei X, Shen K, Shu Y, Wan X, et al. A comparison between drug-eluting bead-transarterial chemoembolization and conventional transarterial chemoembolization in patients with hepatocellular carcinoma: a meta-analysis of six randomized controlled trials. J Cancer Res Ther. 2020; 16(2):243–249. PMID:
32474508.
34. Fonseca AZ, Santin S, Gomes LG, Waisberg J, Ribeiro MA Jr. Complications of radiofrequency ablation of hepatic tumors: frequency and risk factors. World J Hepatol. 2014; 6(3):107–113. PMID:
24672640.
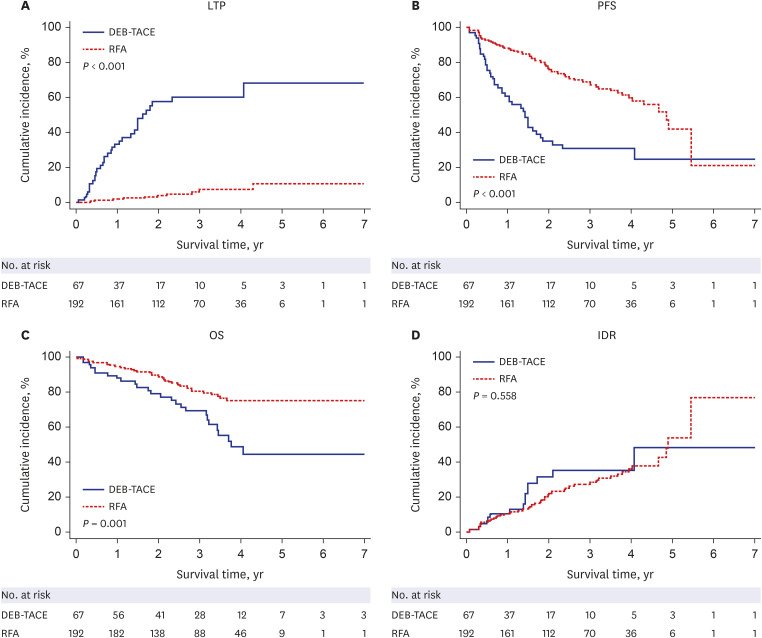
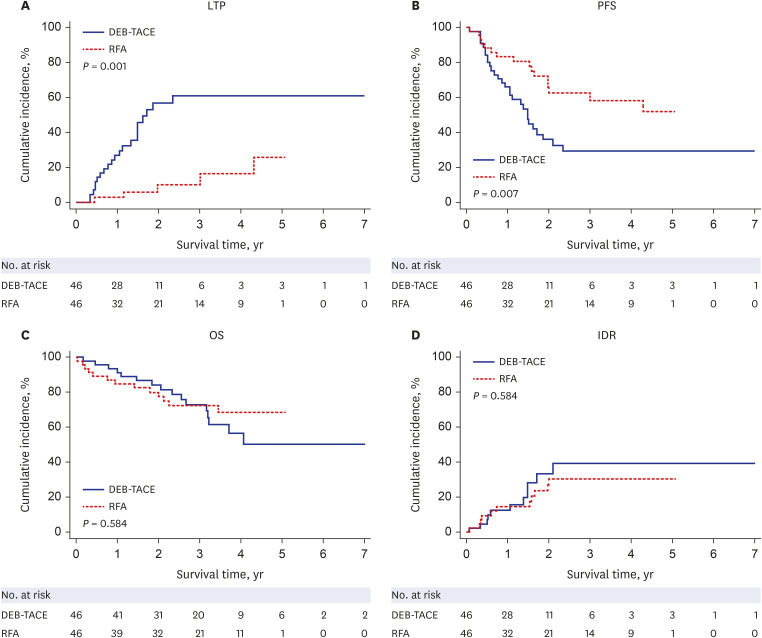




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download