1. Oyler RF, Oyler AL, Matkin ND. Unilateral hearing loss: demographics and educational impact. Lang Speech Hear Serv Sch. 1988; 19(2):201–210.
2. Charabi S, Tos M, Thomsen J, Charabi B, Mantoni M. Vestibular schwannoma growth--long-term results. Acta Otolaryngol Suppl. 2000; 543(543):7–10. PMID:
10908961.
3. Deen HG, Ebersold MJ, Harner SG, Beatty CW, Marion MS, Wharen RE, et al. Conservative management of acoustic neuroma: an outcome study. Neurosurgery. 1996; 39(2):260–264. PMID:
8832662.
4. Hoistad DL, Melnik G, Mamikoglu B, Battista R, O’Connor CA, Wiet RJ. Update on conservative management of acoustic neuroma. Otol Neurotol. 2001; 22(5):682–685. PMID:
11568679.
5. Tschudi DC, Linder TE, Fisch U. Conservative management of unilateral acoustic neuromas. Am J Otol. 2000; 21(5):722–728. PMID:
10993466.
6. Walsh RM, Bath AP, Bance ML, Keller A, Tator CH, Rutka JA. The natural history of untreated vestibular schwannomas. Is there a role for conservative management? Rev Laryngol Otol Rhinol (Bord). 2000; 121(1):21–26. PMID:
10865479.
7. Son EI, Kim IM, Kim SP. Vestibular schwannoma with malignant transformation: a case report. J Korean Med Sci. 2001; 16(6):817–821. PMID:
11748371.
8. Hirsch A, Norén G. Audiological findings after stereotactic radiosurgery in acoustic neurinomas. Acta Otolaryngol. 1988; 106(3-4):244–251. PMID:
3051887.
9. Lee WJ, Lee JI, Choi JW, Kong DS, Nam DH, Cho YS, et al. Optimal volume of the residual tumor to predict long-term tumor control using stereotactic radiosurgery after facial nerve-preserving surgery for vestibular schwannomas. J Korean Med Sci. 2021; 36(16):e102. PMID:
33904259.
10. Kim KM, Park CK, Chung HT, Paek SH, Jung HW, Kim DG. Long-term outcomes of gamma knife stereotactic radiosurgery of vestibular schwannomas. J Korean Neurosurg Soc. 2007; 42(4):286–292. PMID:
19096558.
11. Kondziolka D, Lunsford LD, McLaughlin MR, Flickinger JC. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998; 339(20):1426–1433. PMID:
9811917.
12. Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988; 97(1):55–66. PMID:
3277525.
13. Norén G. Long-term complications following gamma knife radiosurgery of vestibular schwannomas. Stereotact Funct Neurosurg. 1998; 70(Suppl 1):65–73. PMID:
9782237.
14. Yang I, Sughrue ME, Han SJ, Fang S, Aranda D, Cheung SW, et al. Facial nerve preservation after vestibular schwannoma Gamma Knife radiosurgery. J Neurooncol. 2009; 93(1):41–48. PMID:
19430881.
15. Delsanti C, Tamura M, Galanaud D, Régis J. Changing radiological results, pitfalls and criteria of failure. Neurochirurgie. 2004; 50(2-3 Pt 2):312–319. PMID:
15179284.
16. Pollock BE. Management of vestibular schwannomas that enlarge after stereotactic radiosurgery: treatment recommendations based on a 15 year experience. Neurosurgery. 2006; 58(2):241–248. PMID:
16462477.
17. Kwon Y, Khang SK, Kim CJ, Lee DJ, Lee JK, Kwun BD. Radiologic and histopathologic changes after Gamma Knife radiosurgery for acoustic schwannoma. Stereotact Funct Neurosurg. 1999; 72(Suppl 1):2–10. PMID:
10681685.
18. Nakamura H, Jokura H, Takahashi K, Boku N, Akabane A, Yoshimoto T. Serial follow-up MR imaging after gamma knife radiosurgery for vestibular schwannoma. AJNR Am J Neuroradiol. 2000; 21(8):1540–1546. PMID:
11003293.
19. Norén G, Greitz D, Hirsch A, Lax I. Gamma knife surgery in acoustic tumours. Acta Neurochir Suppl (Wien). 1993; 58:104–107. PMID:
8109269.
20. Yu CP, Cheung JY, Leung S, Ho R. Sequential volume mapping for confirmation of negative growth in vestibular schwannomas treated by gamma knife radiosurgery. J Neurosurg. 2000; 93(Suppl 3):82–89.
21. Iwai Y, Yamanaka K, Shiotani M, Uyama T. Radiosurgery for acoustic neuromas: results of low-dose treatment. Neurosurgery. 2003; 53(2):282–287. PMID:
12925242.
22. Flickinger JC, Kondziolka D, Niranjan A, Maitz A, Voynov G, Lunsford LD. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2004; 60(1):225–230. PMID:
15337560.
23. Sakamoto T, Fukuda S, Inuyama Y. Hearing loss and growth rate of acoustic neuromas in follow-up observation policy. Auris Nasus Larynx. 2001; 28(Suppl):S23–S27. PMID:
11683337.
24. Zanoletti E, Mazzoni A, d’Avella D. Hearing preservation in small acoustic neuroma: observation or active therapy? Literature review and institutional experience. Acta Neurochir (Wien). 2019; 161(1):79–83. PMID:
30535851.
25. Zanoletti E, Cazzador D, Faccioli C, Gallo S, Denaro L, D’Avella D, et al. Multi-option therapy vs observation for small acoustic neuroma: hearing-focused management. Acta Otorhinolaryngol Ital. 2018; 38(4):384–392. PMID:
30197430.
26. Flickinger JC, Kondziolka D, Niranjan A, Lunsford LD. Results of acoustic neuroma radiosurgery: an analysis of 5 years’ experience using current methods. J Neurosurg. 2001; 94(1):1–6.
27. Chopra R, Kondziolka D, Niranjan A, Lunsford LD, Flickinger JC. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2007; 68(3):845–851. PMID:
17379451.
28. Flickinger JC, Lunsford LD, Linskey ME, Duma CM, Kondziolka D. Gamma knife radiosurgery for acoustic tumors: multivariate analysis of four year results. Radiother Oncol. 1993; 27(2):91–98. PMID:
8356233.
29. Flickinger JC, Kondziolka D, Niranjan A, Lunsford LD. Results of acoustic neuroma radiosurgery: an analysis of 5 years’ experience using current methods. J Neurosurg. 2001; 94(1):1–6.
30. Flickinger JC, Kondziolka D, Niranjan A, Maitz A, Voynov G, Lunsford LD. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2004; 60(1):225–230. PMID:
15337560.
31. Lunsford LD, Niranjan A, Flickinger JC, Maitz A, Kondziolka D. Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg. 2005; 102(Suppl):195–199. PMID:
15662809.
32. Chung WY, Liu KD, Shiau CY, Wu HM, Wang LW, Guo WY, et al. Gamma knife surgery for vestibular schwannoma: 10-year experience of 195 cases. J Neurosurg. 2005; 102(Suppl):87–96. PMID:
15662787.
33. Myrseth E, Møller P, Pedersen PH, Vassbotn FS, Wentzel-Larsen T, Lund-Johansen M. Vestibular schwannomas: clinical results and quality of life after microsurgery or gamma knife radiosurgery. Neurosurgery. 2005; 56(5):927–935. PMID:
15854240.
34. Chopra R, Kondziolka D, Niranjan A, Lunsford LD, Flickinger JC. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2007; 68(3):845–851. PMID:
17379451.
35. Régis J, Tamura M, Delsanti C, Roche PH, Pellet W, Thomassin JM. Hearing preservation in patients with unilateral vestibular schwannoma after gamma knife surgery. Prog Neurol Surg. 2008; 21:142–151. PMID:
18810212.
36. Yang I, Sughrue ME, Han SJ, Fang S, Aranda D, Cheung SW, et al. Facial nerve preservation after vestibular schwannoma Gamma Knife radiosurgery. J Neurooncol. 2009; 93(1):41–48. PMID:
19430881.
37. Tamura M, Carron R, Yomo S, Arkha Y, Muraciolle X, Porcheron D, et al. Hearing preservation after gamma knife radiosurgery for vestibular schwannomas presenting with high-level hearing. Neurosurgery. 2009; 64(2):289–296. PMID:
19057423.
38. Régis J, Carron R, Park MC, Soumare O, Delsanti C, Thomassin JM, et al. Wait-and-see strategy compared with proactive Gamma Knife surgery in patients with intracanalicular vestibular schwannomas. J Neurosurg. 2010; 113(Suppl):105–111. PMID:
21121792.
39. Murphy ES, Barnett GH, Vogelbaum MA, Neyman G, Stevens GH, Cohen BH, et al. Long-term outcomes of Gamma Knife radiosurgery in patients with vestibular schwannomas. J Neurosurg. 2011; 114(2):432–440. PMID:
20095786.
40. Breivik CN, Nilsen RM, Myrseth E, Pedersen PH, Varughese JK, Chaudhry AA, et al. Conservative management or gamma knife radiosurgery for vestibular schwannoma: tumor growth, symptoms, and quality of life. Neurosurgery. 2013; 73(1):48–56. PMID:
23615094.
41. Kim YH, Kim DG, Han JH, Chung HT, Kim IK, Song SW, et al. Hearing outcomes after stereotactic radiosurgery for unilateral intracanalicular vestibular schwannomas: implication of transient volume expansion. Int J Radiat Oncol Biol Phys. 2013; 85(1):61–67. PMID:
22580122.
42. Carlson ML, Jacob JT, Pollock BE, Neff BA, Tombers NM, Driscoll CL, et al. Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: patterns of hearing loss and variables influencing audiometric decline. J Neurosurg. 2013; 118(3):579–587. PMID:
23101446.
43. Hasegawa T, Kida Y, Kato T, Iizuka H, Kuramitsu S, Yamamoto T. Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: evaluation of 440 patients more than 10 years after treatment with Gamma Knife surgery. J Neurosurg. 2013; 118(3):557–565. PMID:
23140152.
44. Boari N, Bailo M, Gagliardi F, Franzin A, Gemma M, del Vecchio A, et al. Gamma Knife radiosurgery for vestibular schwannoma: clinical results at long-term follow-up in a series of 379 patients. J Neurosurg. 2014; 121(Suppl):123–142.
45. Jacob JT, Carlson ML, Schiefer TK, Pollock BE, Driscoll CL, Link MJ. Significance of cochlear dose in the radiosurgical treatment of vestibular schwannoma: controversies and unanswered questions. Neurosurgery. 2014; 74(5):466–474. PMID:
24476904.
46. Bir SC, Ambekar S, Bollam P, Nanda A. Long-term outcome of gamma knife radiosurgery for vestibular schwannoma. J Neurol Surg B Skull Base. 2014; 75(4):273–278. PMID:
25093151.
47. Watanabe S, Yamamoto M, Kawabe T, Koiso T, Yamamoto T, Matsumura A, et al. Stereotactic radiosurgery for vestibular schwannomas: average 10-year follow-up results focusing on long-term hearing preservation. J Neurosurg. 2016; 125(Suppl 1):64–72. PMID:
27903183.
48. Bowden G, Cavaleri J, Monaco E 3rd, Niranjan A, Flickinger J, Lunsford LD. Cystic vestibular schwannomas respond best to radiosurgery. Neurosurgery. 2017; 81(3):490–497. PMID:
28368501.
49. Tuleasca C, Faouzi M, Maeder P, Maire R, Knisely J, Levivier M. Biologically effective dose correlates with linear tumor volume changes after upfront single-fraction stereotactic radiosurgery for vestibular schwannomas. Neurosurg Rev. 2021; 44(6):3527–3537. PMID:
33839944.
50. Jones B, Hopewell JW, Paddick I. Biologically effective dose correlates with linear tumour volume changes after upfront single-fraction stereotactic radiosurgery for vestibular schwannomas. Neurosurg Rev. 2022; 45(3):2493–2495. PMID:
35290549.
51. Savardekar AR, Terrell D, Lele SJ, Diaz R, Keesari PR, Trosclair K, et al. Primary treatment of small to medium (<3 cm) sporadic vestibular schwannomas: a systematic review and meta-analysis on hearing preservation and tumor control rates for microsurgery versus radiosurgery. World Neurosurg. 2022; 160:102–113.e12. PMID:
34838768.
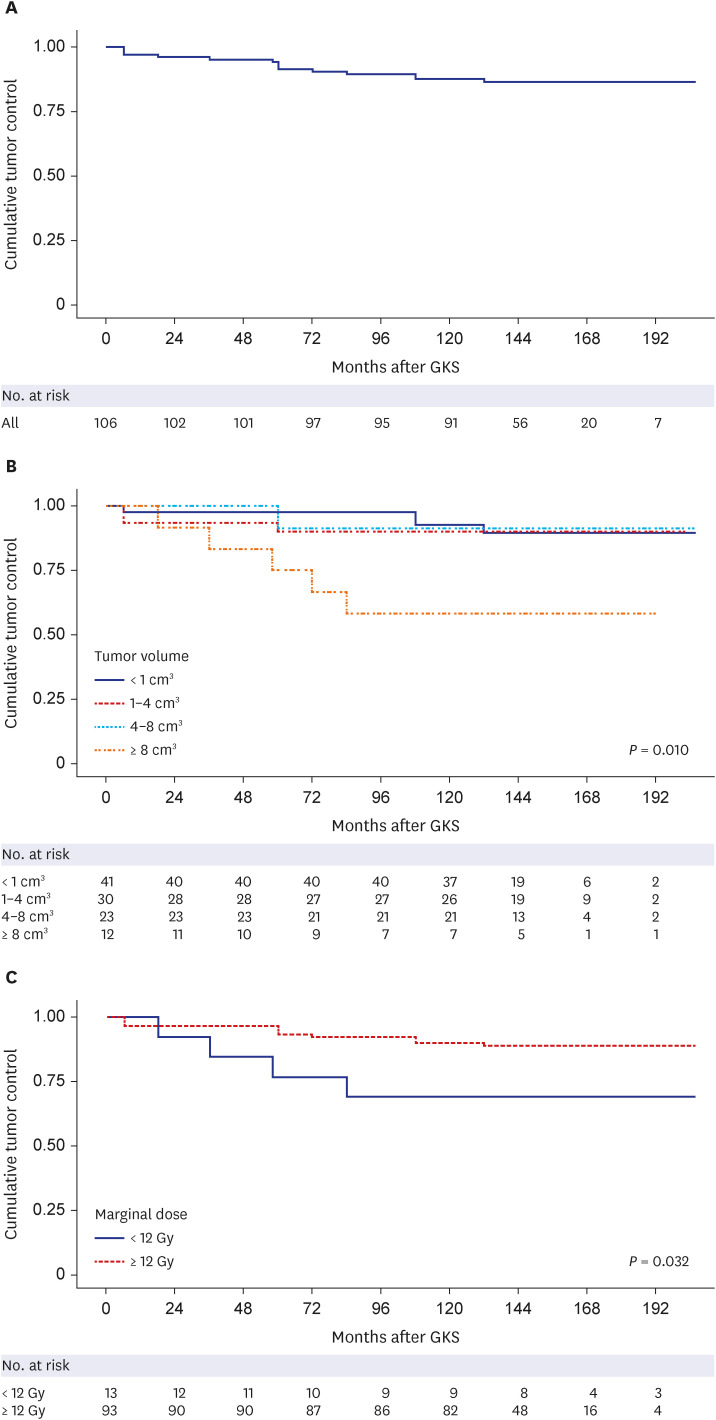
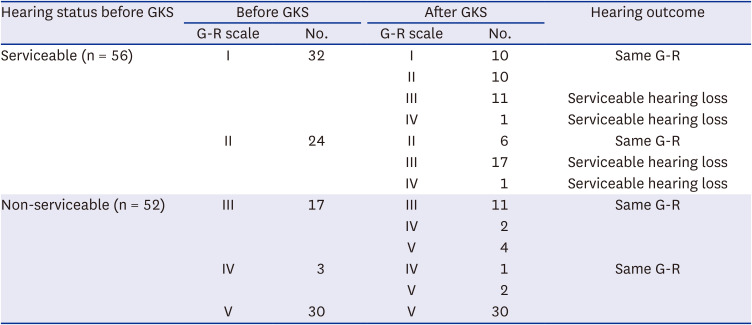
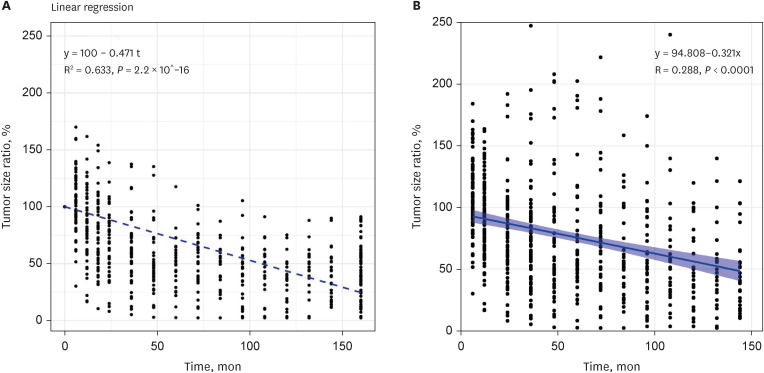
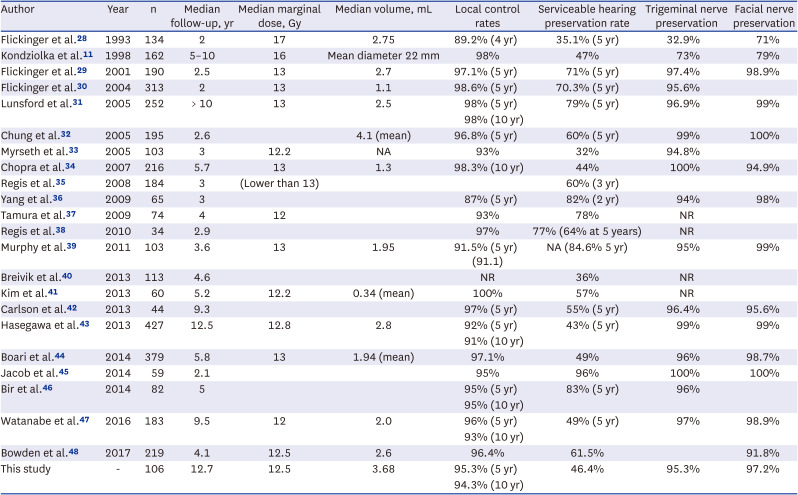




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download