1. Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol. 2012; 13(8):790–801. PMID:
22658655.
2. Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018; 392(10159):2052–2090. PMID:
30340847.
3. Global Burden of Disease 2019 Cancer Collaboration. Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. 2022; 8(3):420–444. PMID:
34967848.
4. Wyld L, Audisio RA, Poston GJ. The evolution of cancer surgery and future perspectives. Nat Rev Clin Oncol. 2015; 12(2):115–124. PMID:
25384943.
5. Sullivan R, Alatise OI, Anderson BO, Audisio R, Autier P, Aggarwal A, et al. Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 2015; 16(11):1193–1224. PMID:
26427363.
6. Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med. 2002; 162(18):2074–2079. PMID:
12374515.
7. Wong TJ, Yu T. Trends in the distribution of body mass index, waist circumference and prevalence of obesity among Taiwanese adults, 1993-2016. PLoS One. 2022; 17(9):e0274134. PMID:
36084122.
8. Olson CM. Nutrition and health outcomes associated with food insecurity and hunger. J Nutr. 1999; 129(2S):Suppl. 521S–4S. PMID:
10064322.
9. Kim YH, Kim SM, Han KD, Jung JH, Lee SS, Oh SW, et al. Waist circumference and all-cause mortality independent of body mass index in Korean population from the National Health Insurance Health Checkup 2009-2015. J Clin Med. 2019; 8(1):72. PMID:
30634601.
10. Liu R, Dang S, Zhao Y, Yan H, Han Y, Mi B. Long-term waist circumference trajectories and body mass index with all-cause mortality in older Chinese adults: a prospective nationwide cohort study. Arch Public Health. 2022; 80(1):94. PMID:
36088350.
11. O’Súilleabháin PS, Sutin AR, Gerstorf D. Body mass index, waist circumference, and mortality risks over 27 years of follow-up in old age. Ann Epidemiol. 2020; 46:20–23. PMID:
32532369.
12. Mullen JT, Davenport DL, Hutter MM, Hosokawa PW, Henderson WG, Khuri SF, et al. Impact of body mass index on perioperative outcomes in patients undergoing major intra-abdominal cancer surgery. Ann Surg Oncol. 2008; 15(8):2164–2172. PMID:
18548313.
13. Lee JH, Park B, Joo J, Kook MC, Kim YI, Lee JY, et al. Body mass index and mortality in patients with gastric cancer: a large cohort study. Gastric Cancer. 2018; 21(6):913–924. PMID:
29651648.
14. Fuchs J, Schellerer VS, Brunner M, Geppert CI, Grützmann R, Weber K, et al. The impact of body mass index on prognosis in patients with colon carcinoma. Int J Colorectal Dis. 2022; 37(5):1107–1117. PMID:
35426079.
15. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the Korean National Health Insurance system. Diabetes Metab J. 2014; 38(5):395–403. PMID:
25349827.
16. Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995; 311(6998):158–161. PMID:
7613427.
17. Damhuis RA, Wijnhoven BP, Plaisier PW, Kirkels WJ, Kranse R, van Lanschot JJ. Comparison of 30-day, 90-day and in-hospital postoperative mortality for eight different cancer types. Br J Surg. 2012; 99(8):1149–1154. PMID:
22718521.
18. Song YJ. The South Korean health care system. Japan Med Assoc J. 2009; 52(3):206–209.
19. Maeda K, Ishida Y, Nonogaki T, Mori N. Reference body mass index values and the prevalence of malnutrition according to the Global Leadership Initiative on Malnutrition criteria. Clin Nutr. 2020; 39(1):180–184. PMID:
30712782.
20. Yoon SH, Kye BH, Kim HJ, Jun KH, Cho HM, Chin HM. Risk of malnutrition after gastrointestinal cancer surgery: a propensity score matched retrospective cohort study. Surg Metab Nutr. 2018; 9(1):16–25.
21. Huang TH, Hsieh CC, Kuo LM, Chang CC, Chen CH, Chi CC, et al. Malnutrition associated with an increased risk of postoperative complications following hepatectomy in patients with hepatocellular carcinoma. HPB. 2019; 21(9):1150–1155. PMID:
30765200.
22. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013; 309(1):71–82. PMID:
23280227.
23. Simillis C, Taylor B, Ahmad A, Lal N, Afxentiou T, Powar MP, et al. A systematic review and meta-analysis assessing the impact of body mass index on long-term survival outcomes after surgery for colorectal cancer. Eur J Cancer. 2022; 172:237–251. PMID:
35797761.
24. Park J, Lee SH, Lee JH, Min JJ, Oh AR, Kim K, et al. Association between high preoperative body mass index and mortality after cancer surgery. PLoS One. 2022; 17(7):e0270460. PMID:
35802728.
25. Newell MA, Bard MR, Goettler CE, Toschlog EA, Schenarts PJ, Sagraves SG, et al. Body mass index and outcomes in critically injured blunt trauma patients: weighing the impact. J Am Coll Surg. 2007; 204(5):1056–1061. PMID:
17481540.
26. Bamgbade OA, Rutter TW, Nafiu OO, Dorje P. Postoperative complications in obese and nonobese patients. World J Surg. 2007; 31(3):556–560. PMID:
16957821.
27. Uhlig C, Neto AS, van der Woude M, Kiss T, Wittenstein J, Shelley B, et al. Intraoperative mechanical ventilation practice in thoracic surgery patients and its association with postoperative pulmonary complications: results of a multicenter prospective observational study. BMC Anesthesiol. 2020; 20(1):179. PMID:
32698775.
28. Hussan H, Gray DM 2nd, Hinton A, Krishna SG, Conwell DL, Stanich PP. Morbid obesity is associated with increased mortality, surgical complications, and incremental health care utilization in the peri-operative period of colorectal cancer surgery. World J Surg. 2016; 40(4):987–994. PMID:
26643515.
29. Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk--a review of the literature. Eur J Clin Nutr. 2010; 64(1):16–22. PMID:
19654593.
30. Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr. 2002; 75(4):683–688. PMID:
11916754.
31. Cerhan JR, Moore SC, Jacobs EJ, Kitahara CM, Rosenberg PS, Adami HO, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014; 89(3):335–345. PMID:
24582192.
32. Wie GA, Cho YA, Kim SY, Kim SM, Bae JM, Joung H. Prevalence and risk factors of malnutrition among cancer patients according to tumor location and stage in the National Cancer Center in Korea. Nutrition. 2010; 26(3):263–268. PMID:
19665873.




 PDF
PDF Citation
Citation Print
Print



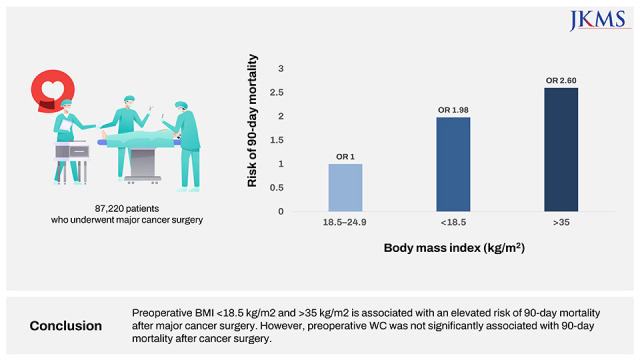
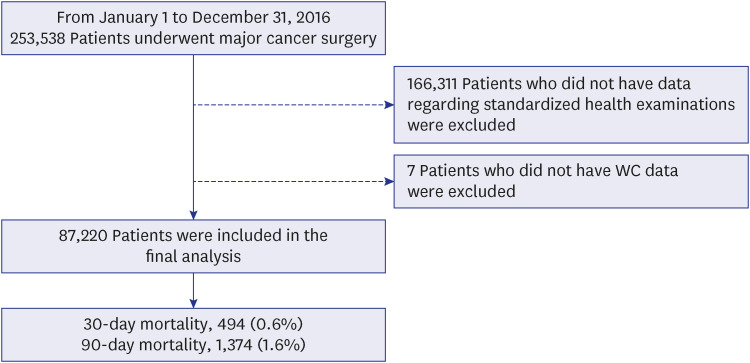
 XML Download
XML Download