Abstract
Purpose
Bacterial infection is a critical complication influencing the survival of a replanted digit. This study aimed to identify risk factors for bacterial infection following zone 1 replantation.
Methods
A retrospective chart review was conducted on patients who underwent zone 1 replantation from January 2016 to November 2022. The factors included in the comparative analysis were patient demographics (age, sex), past medical history (hypertension, diabetes mellitus), smoking, types of injury, degree of contamination, source of trauma, fractures, number of vascular anastomoses, use of salvage therapies, and the use of vein grafts. A bacterial infection was diagnosed based on observation of visible inflammatory signs with the results of culture studies.
Results
In total, 313 patients were selected. Thirty-eight cases of bacterial infection were identified, which accounted for 12.1% of total patients. Methicillin-resistant Staphylococcus epidermidis (MRSE) was the most prevalent bacterium (63.2%, 24 of 38 cases). The patient and injury-related factors showed no significant differences, but the number of vein anastomoses and use of salvage therapy were significantly correlated with the occurrence of bacterial infection.
Conclusion
Performing fewer vein anastomoses appears to increase the likelihood of a salvage procedure, and subsequently increases the risk of bacterial infection by an increased need for direct wound manipulation after zone 1 replantation. Infections caused by MRSE were more commonly identified than those by Aeromonas hydrophilia, which is a commonly known pathogen in medicinal leeches.
Fingertip injuries are common and possess a significant portion of hand trauma cases [1]. In replantation surgery, zone 1 is commonly defined by the distinction at the nail fold. Despite the precise definition can vary, multiple studies concur that it is typically challenging to establish a connection to a dorsal vein [2,3]. While arterial anastomosis tends to yield comparatively good results, venous anastomosis in this area is more difficult to perform, often leading to venous insufficiency after digital replantation [4,5]. Venous insufficiency can lead to blood accumulation, swelling, and the formation of a thrombus within the blood vessel, all of which can potentially cause fingertip necrosis. To overcome this, strategies such as external drainage and medicinal leech therapy are frequently employed [6,7]. The effectiveness of leech therapy in zone 1 digital replantation has been reported in previous study [8]. However, the biggest complication associated with medicinal leech therapy is bacterial infection [9]. It is known that Aeromonas hydrophila, a species of bacteria that parasitizes the gut of leeches, can cause inflammation [10,11]. Precautions should be made and prophylactic antibiotics are recommended for coverage of this bacterial species.
Bacterial infection after zone 1 replantation can result in severe complications and potentially cause the replantation to fail. The purpose of this study is to explore the risk factors for bacterial infection following zone 1 replantation.
Ethics statement: This study was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from the patients for the publication of this study including all clinical images. The study design was approved by the Institutional Review Board of Gwangmyeong Sungae General Hospital (No. KIRB-2023-N-007).
This retrospective study has collected data concerning risk factors associated with bacterial infections following zone 1 replantation from January 2016 to November 2022.
To minimize the risk of infection, all surgeries were performed in the operating room under aseptic conditions. Rigorous irrigation was carried out to reduce contamination of the amputated part before the operation. If bacterial infection occurred, active antibiotics were used to try to slow its progression. The determination of infection was based on the primary physician’s monitoring. If there were signs of erythema, heating sensation, or discharge at the surgical site, bacterial infection was suspected, and proactive measures were taken. In cases of suspected bacterial infection, an immediate wound culture was performed. Exclusion criteria included those with a prior surgical history or trauma, and those with a follow-up period of less than a month. After 3 weeks, the viability was assessed.
In cases of avulsion injury or severe crushing injury leading to substantial arterial damage, debridement was performed up to the healthy vessel lumen before arterial anastomosis. When an arterial gap occurred and made direct end-to-end anastomosis impossible, or it was anticipated that the tension on the artery would be too high, a vein graft was planned. All vein grafts were harvested from the volar aspect of the distal forearm, with careful attention paid to match the diameter of the artery and the graft. Vein anastomosis was not performed when the diameter of the vessel was too small due to zone 1 distal tip amputation, or when a suitable vessel for anastomosis could not be found. Vein-to-vein graft anastomosis was not considered.
Detailed data were collected on potential risk factors for bacterial infection, including patient demographics (age, sex), past medical illness (hypertension, diabetes mellitus), smoking, injury types, degree of contamination, trauma source, the presence of fractures, number of vascular anastomoses, the use of salvage therapies, and the use of vein graft.
Based on the amputation mechanism, injury patterns were categorized into three groups. Group A included cases characterized by cleanly cut surfaces. Group B involved cases with partial crushing and pinpoint hemorrhaging visible on the skin at the cut site. Group C comprised cases with severe crushing exhibiting a red line sign suggestive of vessel damage (Fig. 1). Degree of contamination was categorized on a scale from 1 to 3: grade 1 entailed no visible contaminants on the cut surface; grade 2 involved visible contaminants affecting less than 50% of the cut surface; and grade 3 consisted of visible contaminants invading more than 50% of the cut surface (Fig. 2).
Postoperatively, patients were instructed to rest in bed for 1 week with their hands elevated and exposed to infrared light. All patients were prescribed intravenous administration of first-generation cephalosporin (cefazedone sodium 1 g, three times a day) and aminoglycoside (gentamicin sulfate 80 mg, once a day) for 5 days. This was followed by an 8-day course of first-generation cephalosporin (cefazedone sodium 1 g, twice a day) to provide coverage against both gram-positive and gram-negative organisms. In cases where patients exhibited cephalosporin allergies, an alternative regimen of intravenous aminoglycoside (gentamicin sulfate 80 mg, three times a day for the first 5 days and once a day for the subsequent 8 days) was administered. Additionally, intravenous injections of 2,000 units of heparin were given every 4 hours, and prostaglandin E1 (Eglandin; Mitsubishi Tanabe Pharma Inc., Osaka, Japan) was concurrently administered at a daily dose of 10 µg/kg for 5 days.
The circulation of the replantation site was monitored hourly for the first 3 days after surgery and every 4 hours for the following 4 days. All patients received treatment after being admitted to the inpatient ward. Evaluation of venous congestion was proceeded by observing the signs of color changes and capillary refilling time [12]. Dressings were changed whenever necessary due to excessive bleeding.
In cases of congestion, bleeding was promptly induced by creating a full-thickness defect in the fingertip and stimulating the external wound with gauze soaked in heparinized saline. If congestion was not fully resolved, medicinal leeches (Hirudo medicinalis; Biopharm, Hendy, UK ) were used to induce further bleeding through the external wound.
Signs of inflammatory changes and circulation were closely monitored. Upon detecting signs of infection, immediate wound culture studies were initiated. Specimens were cultured from the infection site using transport swabs (Copan Diagnostics, Murrieta, CA, USA). Bacterial identification was conducted on MacConkey and sheep blood agar plates. Antibiotic sensitivity testing and staining confirmation proceeded via microbial identification methods. Consultations with the infectious disease department guided any necessary adjustments to the type and duration of antibiotic usage.
Statistical significance between continuous variables of the two groups was analyzed by the Mann-Whitney test, while discrete variables were analyzed by the chi-square test. A p-value less than 0.05 was considered statistically significant. Statistical analysis was conducted using IBM SPSS Statistics ver. 26.0 (IBM Corp., Armonk, NY, USA).
A retrospective analysis was conducted on a total of 313 patients. The study found an overall survival rate of 86.3% (270 out of 313 cases). Among these cases, 38 were identified with bacterial infection. The survival rate of replantation in the group with bacterial infection was determined to be 65.8% (25 out of 38 cases). In contrast, the group without bacterial infection exhibited a survival rate of 89.1% (245 out of 275 cases). The occurrence of bacterial infection showed a statistically significant difference in survival rates (p=0.039) (Table 1).
No statistically significant differences were observed between the two groups—those with and without bacterial infection—with regard to patient factors, including sex, age, underlying diseases (hypertension and diabetes mellitus), and smoking status (Table 2).
Factors related to the initial trauma event, such as injury type, presence of fracture, degree of contamination, and trauma source, did not exhibit any statistically significant correlation with each other (Table 3).
Upon examination of surgical factors, the number of vein anastomoses displayed a statistically significant difference (p=0.031) between groups with and without bacterial infection. Specifically, 52.6% (20 of 38 cases) of the bacterial infection group exhibited no vein anastomoses, contrasted with 37.8% (104 of 275 cases) in the group without bacterial infection. Moreover, 26.3% (10 of 38 cases) of the infection group had one vein anastomosis, as compared to 43.6% (120 of 275 cases) in the non-infection group. For two vein anastomoses, 15.8% (6 of 38 cases) were observed in the bacterial infection group, while the non-infected group accounted for 15.4% (42 of 275 cases). Three venous anastomoses were found in 5.3% (2 out of 38 cases) of the bacterial infection group and 3.3% (9 out of 275 cases) of the non-infection group.
Salvage therapy also exhibited a significant difference (p=0.005) between the two groups. Within the bacterial infection group, 21.1% (8 of 38 cases) underwent external bleeding therapy, 2.6% (1 of 38 cases) received leech therapy, 28.9% (11 of 38 cases) underwent both external bleeding and leech therapy, and 47.4% (18 of 38 cases) had no salvage therapy. In contrast, the non-infection group experienced rates of 11.4% (31 of 275 cases) for external bleeding therapy, 3.3% (9 of 275 cases) for leech therapy, 16.3% (45 of 275 cases) for both therapies, and 69.1% (190 of 275 cases) for no salvage therapy. Neither the use of vein grafts nor the number of artery anastomoses demonstrated statistically significant differences (Table 4). Among 38 confirmed bacterial infection cases, methicillin-resistant Staphylococcus epidermidis (MRSE) was the most frequently detected organism (63.2%) (Fig. 3).
A 71-year-old female patient was admitted to our hospital after her finger got caught and twisted in a spinning washing machine. Zone 1 replantation was performed on the injured finger, during which a single vein, measuring 0.5 mm in diameter, was successfully anastomosed. After 4 days, the patient began showing signs of congestion, which led to the initiation of 5-day salvage therapy. On postoperative day 10, a wound culture test confirmed the presence of MRSE. An antibiotic regimen was administered to prevent necrosis of the replanted digit due to bacterial infection. The patient showed improved inflammatory signs without the need for further surgery and was discharged on postoperative day 29 (Fig. 4).
This study provides a comparative evaluation of risk factors contributing to bacterial infection in patients undergoing zone 1 replantation. It is recognized that contamination by organic material, as in barnyard injuries, can increase postoperative wound infection risk, potentially leading to replantation failure and systemic sequelae [13]. Our study found no significant association between patient factors, trauma factors, and bacterial infection occurrence. This suggests that, despite severe contamination and organic material-induced injuries, proper surgical procedures, including thorough debridement and contaminant removal, can reduce bacterial infection rates.
Furthermore, a previous study conducted in our institute has stated that the presence of crushing did not affect the survival rate in Tamai zone I replantation [14]. This study also confirmed that the presence of crushing injuries does not have a significant impact on the incidence of bacterial infection, which can influence the survival rate and prognosis of zone 1 replantation. Therefore, surgeons can use these findings to help determine whether or not to proceed with replantation.
A number of previous studies have emphasized the importance of venous anastomosis in digital replantation [15,16]. Insufficient venous anastomosis can lead to higher venous congestion incidence. Ryu et al. [17] have stated that a greater number of venous anastomoses was associated with a decreased need for external bleeding, corresponding to a significant decrease in the need for postoperative monitoring and leech therapy. Although salvage therapy can be a straightforward and effective approach in artery-only zone 1 replantation, it poses a high risk of potential complications, ranging from the necessity of blood transfusions to the occurrence of bacterial infections [18]. Venous congestion may necessitate the implementation of salvage therapy, which typically involves increased direct wound manipulation. Consequently, this elevates the chance of bacterial infection. Therefore, we believe that investing additional time and effort during the initial surgery to perform as much vein anastomosis as possible, even if it proves challenging, could prevent venous congestion and decrease the necessity for salvage therapy. In turn, this approach could not only prevent bacterial infections and improve digit survival but also avoid other complications, ultimately contributing to an enhanced survival rate.
Ever since Foucher et al. [19] reported successful results using medicinal leeches (Hirudo medicinalis) to alleviate venous congestion after a digital replantation where venous anastomosis was not possible, the use of medicinal leeches has been generalized for the purpose of relieving venous congestion following flap surgery or microsurgery. In 1983, Whitlock et al. [20] revealed that A. hydrophila, a bacterium residing in the intestines of leeches, is a human pathogen. Subsequently, Mercer et al. [21] reported that about 20% of patients who were treated with medicinal leeches were confirmed to have A. hydrophila infections. In our study, however, we observed infections caused by MRSE more frequently than those found in the intestines of leeches. MRSE is one of the key drug-resistant bacteria in infected wounds from trauma [22,23]. Effective infection control plays a crucial role in promoting healing and facilitating speedy recovery in these cases [24]. S. epidermidis identified in cultures should not always be dismissed as mere contamination. Appropriate therapeutic measures and preventive guidelines need to be enforced against this concerning pathogen [25]. Thus, when treating bacterial infection following zone 1 replantation, it is reasonable to consider the potential for MRSE infection. Contemplating the use of empirical antibiotics to address MRSE could also prove advantageous when a bacterial infection is suspected after zone 1 replantation.
Furthermore, medical and nursing interventions are a very main role in the transmission of MRSE [26]. Given that S. epidermidis is a commensal organism found on human skin, the importance of strict aseptic techniques during salvage procedures cannot be overemphasized. Salvage therapies, including leech therapy, can inadvertently increase the risk of infection due to the increased handling of the surgical site and the creation of open wounds susceptible to external contamination. Therefore, rigorous maintenance of sterile conditions during such interventions is paramount. This could significantly mitigate the incidence of postoperative infections, consequently improving the surgical outcomes of zone 1 replantation.
However, we were unable to ascertain whether the identified infections were opportunistic, originating from the normal skin flora, or were attributable to nosocomial pathogens. Nosocomial infections are defined as those where previously absent bacteria are detected more than 48 hours after hospital admission [27]. Given that our study did not encompass an examination of the wound culture at the point of patient admission, the precise origin of the infection remained indeterminable. According to the previous study, epidemiological analyses using multilocus sequence typing and genetic studies indicate that S. epidermidis isolates in the hospital environment differ from those obtained outside of medical facilities in terms of biofilm formation, antibiotic resistance, and the presence of mobile DNA elements [28]. This indicates that the characteristics of bacterial pathogens involved in zone 1 replantation can diverge significantly depending on the source of infection, and this variability may in turn influence the appropriate therapeutic approach. Subsequent research should be directed towards distinguishing between infections of nosocomial origin and those attributable to the patient's own microbial flora.
This study has several limitations. Firstly, the analysis did not account for potential variability in surgeon skills, which could have influenced the outcomes. Secondly, the research did not investigate the methods used to preserve and transport the amputated digits, both of which could potentially affect the risk of infection. Thirdly, the study did not evaluate the correlation between the ischemic time of the amputated digits and the survival rate of the replantation; the impact of prolonged ischemic times on the survival rate of replantation remained unexplored. When patients requiring replantation presented to the emergency room, the initial examination record did not include inquiries about ischemic time, the method of preserving the amputated part, or the method of transport. If these items are added to the initial emergency room consultation and subsequent research investigates these aspects, it appears that the research results could be significantly enhanced. Finally, the study lacked sufficient indicators and research data concerning the incidence of contact-related infections during salvage therapy.
Bacterial infection significantly reduced the survival rate of zone 1 replantation, with MRSE being the most frequently isolated pathogen. Performing more vein anastomoses significantly decreased the incidence of bacterial infection, potentially by reducing the need for salvage therapy, which independently influences the risk of bacterial infection. When a bacterial infection is suspected, it may be beneficial to consider covering for MRSE species in the therapeutic regimen.
References
1. Sebastin SJ, Chung KC. A systematic review of the outcomes of replantation of distal digital amputation. Plast Reconstr Surg. 2011; 128:723–37.

2. Komatsu S, Tamai S. Successful replantation of a completely cut-off thumb. Plast Reconst Surg. 1968; 42:374–7.

3. Yamano Y. Replantation of the amputated distal part of the fingers. J Hand Surg Am. 1985; 10:211–8.

4. Buntic RF, Brooks D. Standardized protocol for artery-only fingertip replantation. J Hand Surg Am. 2010; 35:1491–6.

5. Chen KK, Hsieh TY, Chang KP. Tamai zone I fingertip replantation: is external bleeding obligatory for survival of artery anastomosis-only replanted digits? Microsurgery. 2014; 34:535–9.

6. Barnett GR, Taylor GI, Mutimer KL. The "chemical leech": intra-replant subcutaneous heparin as an alternative to venous anastomosis. Report of three cases. Br J Plast Surg. 1989; 42:556–8.

7. Foucher G, Norris RW. Distal and very distal digital replantations. Br J Plast Surg. 1992; 45:199–203.

8. Kim SW, Han HH, Jung SN. Use of the mechanical leech for successful zone I replantation. ScientificWorldJournal. 2014; 2014:105234.

9. Lee NH, Yang KM. The problem of leech application in digital replantation. J Korean Soc Surg Hand. 2000; 9:158–63.
10. Busing KH, Doll W, Freytag K. Bacterial flora of the medicinal leech. Arch Mikrobiol. 1953; 19:52–86.
11. Daane S, Zamora S, Rockwell WB. Clinical use of leeches in reconstructive surgery. Am J Orthop (Belle Mead NJ). 1997; 26:528–32.
12. Prsic A, Friedrich JB. Postoperative management and rehabilitation of the replanted or revascularized digit. Hand Clin. 2019; 35:221–9.

13. Boulas HJ. Amputations of the fingers and hand: indications for replantation. J Am Acad Orthop Surg. 1998; 6:100–5.
14. Hong MK, Park J, Koh SH, et al. Analysis of outcomes of Tamai zone I digital replantation in cases of severe crushing injury. Arch Hand Microsurg. 2020; 25:297–303.
15. Hattori Y, Doi K, Ikeda K, Abe Y, Dhawan V. Significance of venous anastomosis in fingertip replantation. Plast Reconstr Surg. 2003; 111:1151–8.
16. Efanov JI, Rizis D, Landes G, Bou-Merhi J, Harris PG, Danino MA. Impact of the number of veins repaired in short-term digital replantation survival rate. J Plast Reconstr Aesthet Surg. 2016; 69:640–5.
17. Ryu DH, Roh SY, Kim JS, Lee DC, Lee KJ. Multiple venous anastomoses decrease the need for intensive postoperative management in tamai zone I replantations. Arch Plast Surg. 2018; 45:58–61.

18. Han SK, Lee BI, Kim WK. Topical and systemic anticoagulation in the treatment of absent or compromised venous outflow in replanted fingertips. J Hand Surg Am. 2000; 25:659–67.

19. Foucher G, Merle M, Braun JB. Distal digital replantation-one of the best indications for microsurgery. Int J Microsurg. 1981; 3:263–70.
20. Whitlock MR, O'Hare PM, Sanders R, Morrow NC. The medicinal leech and its use in plastic surgery: a possible cause for infection. Br J Plast Surg. 1983; 36:240–4.

21. Mercer NS, Beere DM, Bornemisza AJ, Thomas P. Medical leeches as sources of wound infection. Br Med J (Clin Res Ed). 1987; 294:937.

23. Tran PA, O'Brien-Simpson N, Palmer JA, et al. Selenium nanoparticles as anti-infective implant coatings for trauma orthopedics against methicillin-resistant Staphylococcus aureus and epidermidis: in vitro and in vivo assessment. Int J Nanomedicine. 2019; 14:4613–24.
24. Hospenthal DR, Murray CK, Andersen RC, et al. Guidelines for the prevention of infection after combat-related injuries. J Trauma. 2008; 64(3 Suppl):S211–20.
25. Haque N, Bari MS, Haque N, et al. Methicillin resistant Staphylococcus epidermidis. Mymensingh Med J. 2011; 20:326–31.
26. Kalani BS, Khodaei F, Moghadampour M, et al. TRs analysis revealed Staphylococcus epidermidis transmission among patients and hospital. Ann Ig. 2019; 31:52–61.
Fig. 1.
(A) Group A, clean-cut injury. (B) Group B, partial crushing with petechiae on the skin. (C) Group C, severe crushing with a red line sign suggesting vessel damage.
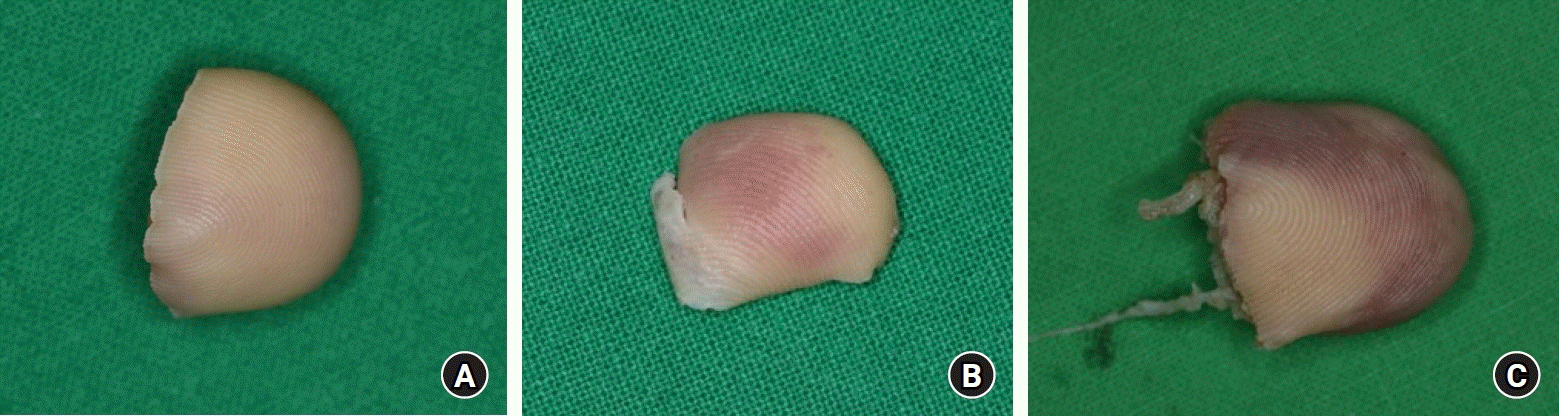
Fig. 2.
Classification of the degree of contamination. (A) Grade 1 with no visible contamination. (B) Grade 2 with visible contamination on less than 50% of the cut surface. (C) Grade 3 with visible contamination on more than 50% of the cut surface.
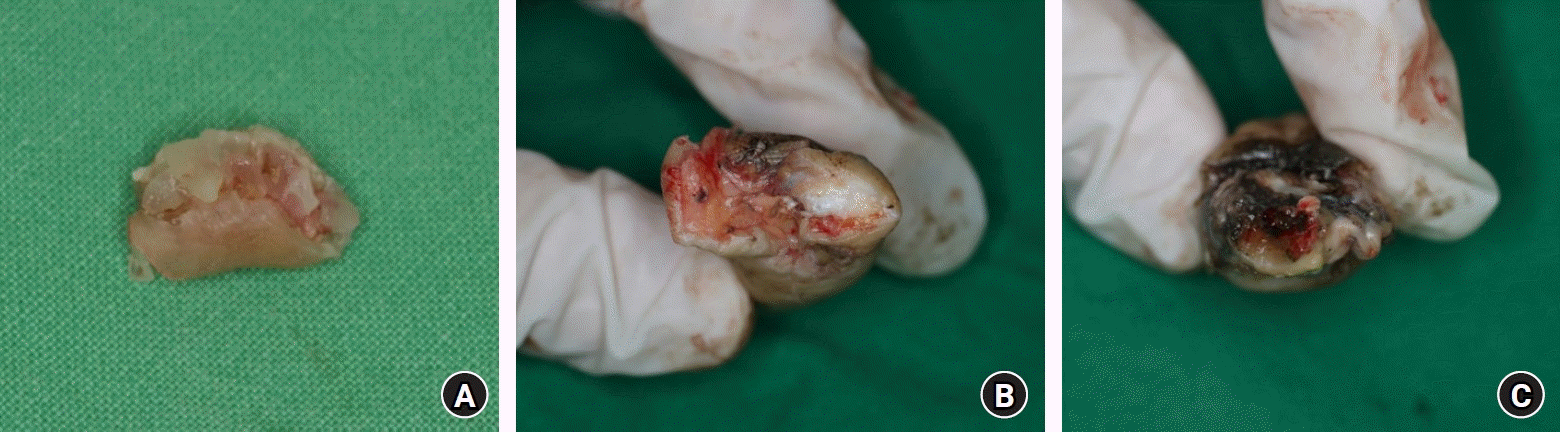
Fig. 3.
Results of bacterial wound cultures. Pie chart indicating the wound cultures of zone 1 replantation. MRSA, methicillin-resistant Staphylococcus aureus; MRSE, methicillin-resistant Staphylococcus epidermidis.
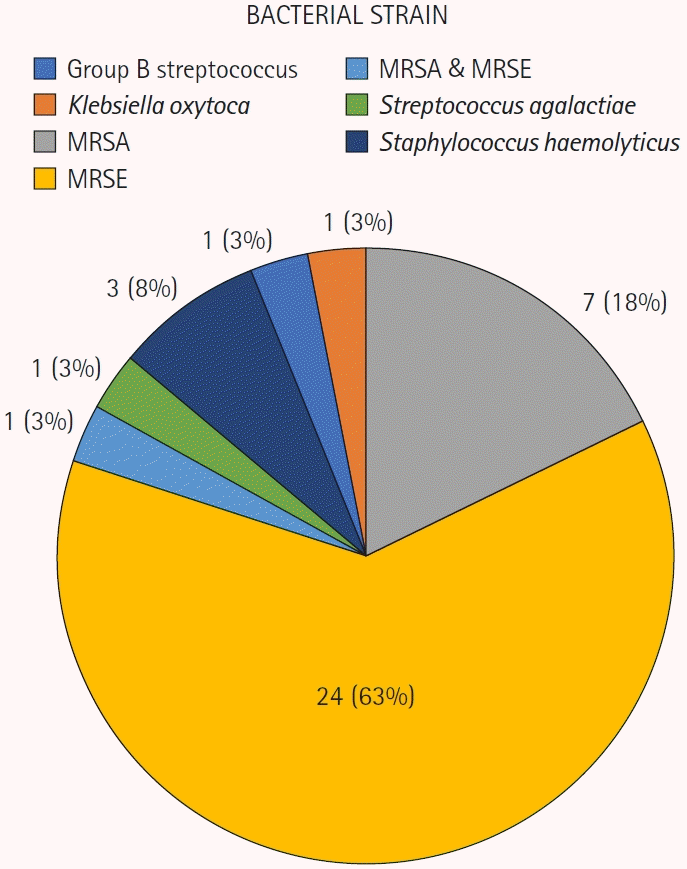
Fig. 4.
(A) Preoperative photograph. (B) Immediate postoperative photograph. (C) Observation of a bacterial infection on postoperative day 7. (D) Follow-up photograph on postoperative day 29.
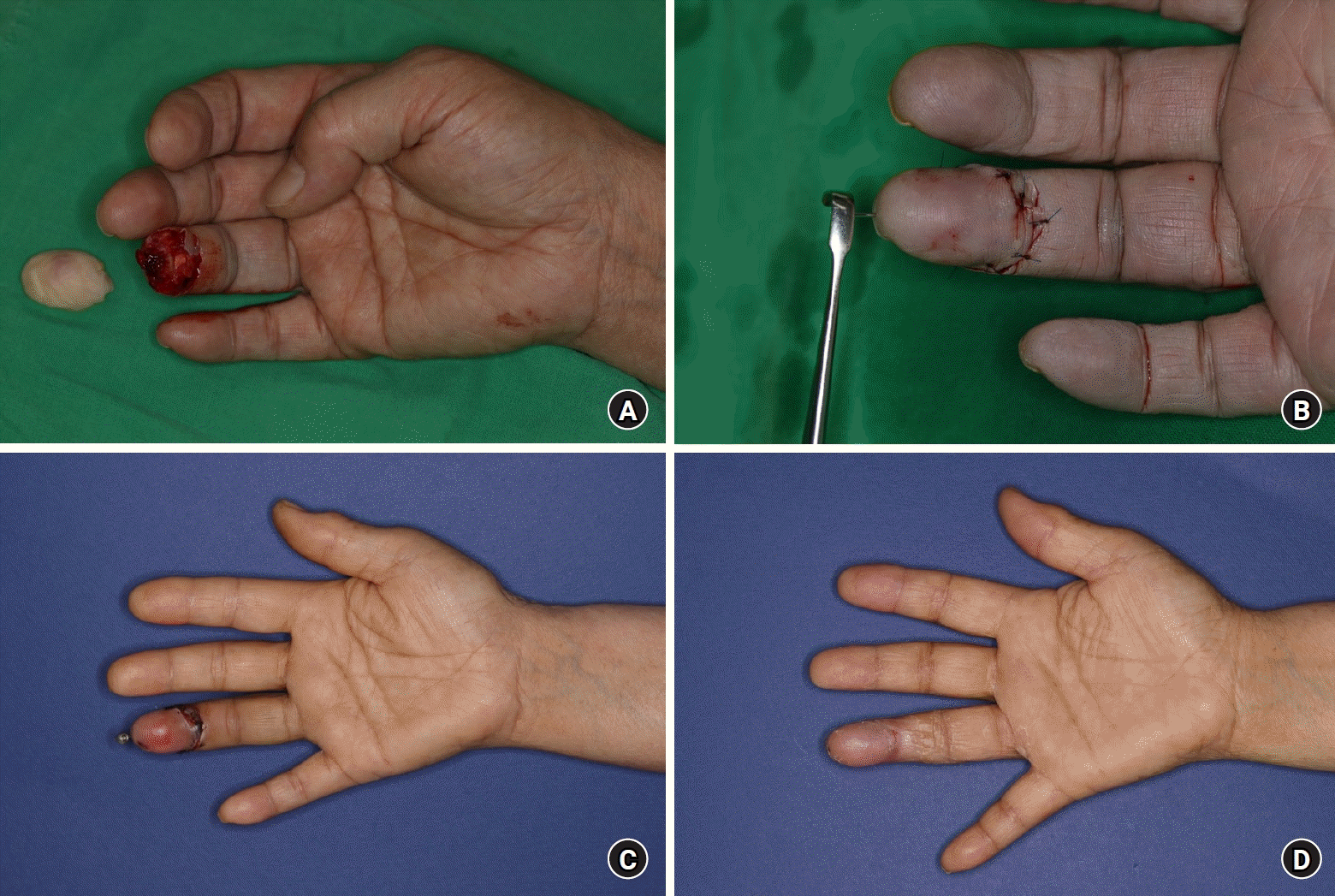
Table 1.
Relationship between postoperative bacterial infection and the survival rate
| Infection | Survival rate | p-value |
|---|---|---|
| Bacterial | 25/38 (65.8) | 0.039 |
| No bacterial | 245/275 (89.1) |
Table 2.
Relationship between postoperative bacterial infection and baseline demographic variables
Table 3.
Relationship between postoperative bacterial infection and clinical variables
Table 4.
Analysis of surgical factors of Tamai zone 1 amputation after replantation in relation to bacterial infection




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download