This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Supraglottic airways (SGA) are increasingly used in pediatric anesthesia. Among SGA, I-gelTM is a commonly used device in pediatric patients. The BlockbusterTM laryngeal mask airway (LMA) is latest addition in pediatric airway armamentarium. This study was conducted to compare the clinical performance of I-gelTM and BlockbusterTM LMA in pediatric patients.
Methods
A total of 140 children aged 1–5 years, who were undergoing elective surgery, were randomized into two groups either I-gelTM (Group I) or BlockbusterTM LMA (Group B). Airway was secured with appropriate-sized LMA according to group allocation under general anesthesia. The primary objective of study was oropharyngeal leak pressures (OPLP), and secondary objectives were number of attempts of device insertion, success rate, ease of LMA insertion, hemodynamic parameters, and postoperative pharyngolaryngeal morbidities.
Results
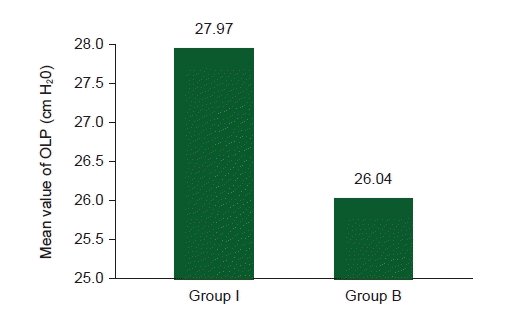
The mean OPLP was significantly higher for I-gelTM compared to BlockbusterTM LMA (27.97 ± 1.65 vs. 26.04 ± 2.12; P < 0.001). The devices were successfully inserted on the first attempt in 97.14% and 90% of the Group I and Group B respectively. The insertion time, ease of insertion, hemodynamic parameters and postoperative complications were comparable between groups.
Conclusions
The I-gelTM was more efficacious device in term of OPLP than BlockbusterTM LMA for positive pressure ventilation in pediatric patients undergoing short surgical procedures under general anesthesia.
Keywords: Blockbuster LMA, I-gel, Pediatric anesthesia, Supraglottic airways
INTRODUCTION
Airway management is a challenging task in children and a variety of devices have been developed precisely for this purpose. A safer alternative to endotracheal intubation was brought to the fore by Archie Brain, namely a supraglottic airway (SGA) [
1]. The advent of SGAs has reduced perioperative airway-related side effects and they have become a more popular device for securing the airway, as reported by the National Audit Project-4 (NAP-4) [
2].
Pediatric-size SGAs are limited, and these devices have been innovated and modified from their adult counterparts, although their efficacy and safety in this subset of the population are limited [
3]. A network meta-analysis on the use and evaluation of different SGAs in pediatric patients concluded that I-gel
TM (Intersurgical Ltd., UK) is one of the most studied SGAs in children with a high oropharyngeal leak pressure (OPLP) and the lowest risk of blood staining among 16 SGAs studied [
4]. Another second-generation laryngeal mask airway (LMA) is Blockbuster
TM LMA (Touren Medical Instrument Co., Ltd., China), invented in 2012 by Professor Ming Tian, made of silicone with an inflatable cuff [
5]. Till now no study was available in literature that compared efficacy and safety of these devices in pediatric population.
Therefore, we designed this study to compare the clinical performance of BlockbusterTM LMA with that of I-gelTM in pediatric patients with the hypothesis that LMA-Blockbuster would have comparable efficacy to I-gel when used in pediatric patients.
METHODS
This study was conducted in tertiary care centre after approval from Institutional Ethics Committee (no. SNMC/IEC/2020/Plan/313) and registration in the Clinical Trial Registry of India (no. CTRI/2020/09/028079). Informed written consent was taken from the parents of all patients. Children of age 1–5 years, weighing between 5 and 25 kg, belonging to the American Society of Anesthesiologists- physical status I or II, scheduled for elective surgery under general anesthesia were included in this study. The syndromic babies, upper respiratory tract infections, silicone allergy, emergency surgery, abnormal anatomy of pharynx and larynx, those at increased risk of aspiration and patients who have received oxygen support or mechanical ventilation in the past one month were excluded from the study.
Children were randomized to either group- the I-gelTM group (Group I) or the BlockbusterTM LMA group (Group B) using a computer-generated random number table. To ensure the confidentiality of the assignment, random numbers were placed in a sealed opaque envelope which was opened upon the child’s arrival in the operating room.
Patients were kept nil per oral as per standard fasting guidelines. Monitoring consisted of electrocardiography, non-invasive blood pressure (NIBP), and pulse oximetry, and baseline vital parameters were recorded. Children were premedicated with midazolam 0.05 mg/kg intravenously (IV), and anesthesia was induced with fentanyl 2 µg/kg and and propofol 2–3 mg/kg IV. Intravenous atracurium 0.5 mg/kg was administered after confirmation of satisfactory mask ventilation. The airway was secured with one of the airway devices as per group allocation. The airway device size was chosen according to body weight and manufacturer recommendations. The lubricated device was inserted in a neutral head position. Both these devices were inserted along the hard palate with the airway device shafts held approximately parallel to the patient’s chest until resistance was felt. The BlockbusterTM LMA cuff was inflated with the appropriate amount of air according to the manufacturer’s instructions. The ventilator was attached to the device and effective placement was assessed by bilateral equal chest movements, square wave capnograph, absence of gastric insufflations epigastrium auscultation and delivery of adequate tidal volume. The insertion time was calculated as the time from picking up the device to the appearance of the first capnographic waveform on the monitor. The number of insertion attempts was also calculated. Insertion failure was marked if the airway could not be secured in three attempts and the patient was intubated via direct laryngoscopy with an appropriate size endotracheal tube. The primary outcome of the study was the comparison of the OPLP and secondary outcomes were insertion parameters such as ease of insertion, time of insertion and number of attempts, as well as hemodynamic changes and incidence of postoperative complications.
The OPLP was determined one minute after securing the airway by closing the circle system’s expiratory valve at a fixed gas flow of 3 L/min. The airway pressure at which equilibrium was reached and a gas leak occurs as determined by an audible leak or by detection of an audible noise with a stethoscope placed directly lateral to the thyroid cartilage was the OPLP [
6].
Ease of insertion was assessed by an objective rating depending on the number of airway manipulations required to introduce the LMA with no manipulation, only one manipulation and more than one manoeuver rated as very easy, easy and difficult respectively. Hemodynamic parameters including heart rate, NIBP and peripheral oxygen saturation (SpO2) were recorded at baseline, immediately after device insertion and every 5 min until surgery was completed. Anesthesia was maintained with sevoflurane in an O2-air mixture with a targeted FiO2 of 40%. Anesthetics were discontinued at the end of the operation; 0.05 mg/kg neostigmine was administered together with 0.01 mg/kg glycopyrrolate to reverse the effect of the neuromuscular blocking agent. Upon return of adequate spontaneous breathing and muscle strength, the device was removed as soon as the child was awake. The device was examined for blood stains and the child was evaluated for other postoperative complications.
All patients' SGAs were inserted by anesthesiologists, who had at least 3 years of SGAs insertion experience or had at least 50 SGAs insertions before the start of the study. The OPLP, SGA insertion time, hemodynamic parameters, and postoperative complications were noted and recorded by an independent observer who was unaware of the inserted device.
The sample size was calculated on previous study by Kim et al. [
7] The oropharyngeal leak pressure for I-gel
TM was mean ± SD; 27.1 ± 6.1 cmH
2O. Assuming a minimum difference of 3 cmH
2O to be clinically significant, the minimum sample size calculated to be 66 in each group at type I error of 0.05 and power of 80%. To account for potential dropouts, we enrolled 70 patients in each group.
The Kolmogorov–Smirnov test was used to determine the distribution of all continuous variables. An independent t-test was used for the normally distributed variables. Fisher’s exact test and chi-square test were used for comparison of qualitative data. The continuous variables were described in mean ± SD, while categorical variables were described in numbers and percentages. Differences were considered significant at P < 0.05.
RESULTS
A total of 149 patients were evaluated for eligibility, of which 5 patients were excluded due to symptoms of upper respiratory tract infection on the day of surgery and the parents of 4 children refused to participate, so the remaining 140 patients were included in the final analysis. Selected children were randomly assigned to Group I and Group B (
Fig. 1). Children included in both groups had comparable demographic variables (
Table 1).
The mean OPLP was significantly higher for I-gel
TM (27.97 ± 1.65) than for Blockbuster
TM LMA (26.04 ± 2.12) (P < 0.001) (
Fig. 2). Total insertion time was comparable between I-gel
TM and Blockbuster
TM LMA at 15.51 ± 1.62 and 15.92 ± 3.02 s respectively. Both groups were found to be comparable in terms of ease of SGA insertion. The devices were successfully inserted on the first attempt in 97.14% and 90% of the I-gel
TM group and Blockbuster
TM LMA groups respectively (
Table 2). All hemodynamic parameters and cases of postoperative complications were comparable between both groups.
DISCUSSION
The main results of the study show that both I-gelTM and BlockbusterTM LMA provide adequate sealing pressure around the laryngeal inlet. However, the I-gelTM provides a comparatively better airway seal than the BlockbusterTM LMA.
Effective and adequate sealing around the glottis becomes important when SGAs are used during surgery to prevent loss of tidal volume, avoidance of operative room air contamination, repeated airway switching and reduce the risk of regurgitation. To ensure adequate ventilation, an ideal SGA should have an OPLP higher than ventilation airway pressure or greater than 20 cmH
2O [
8]. In the literature pediatric counterparts of various first-generation SGA such as classic LMA, flexible LMA, LMA unique, etc. have reported OPLP of 16 to 20 cmH
2O [
9]. Second-generation devices such as the LMA Proseal, Air-Q, I-gel
TM, and LMA Supreme are also available in pediatric sizes and have better seal pressure compared to first-generation devices [
7,
9–
11].
In our study, the OPLP of Blockbuster
TM LMA has been reported to be lower than I-gel
TM (26.04 ± 2.12 mmHg and 27.97 ± 1.65 mmHg respectively), although both these devices provide adequate seal pressure when used for positive pressure ventilation. I-gel
TM has a non-inflatable thermoplastic polymer cuff that is known to conform itself to the glottis to provide an effective seal around the glottis. The cuff is said to respond to body temperature to create an adequate seal around the glottis to resist leakage during positive pressure ventilation [
11,
12]. With the Blockbuster
TM LMA, the 95-degree angled breathing tube and the cuff shape of the Blockbuster
TM LMA can be responsible for the high sealing pressure [
5]. In most studies, the OPLP of I-gel
TM was reported to be greater than 20 cmH
2O in pediatric patients [
13–
15]. While studies using Blockbuster
TM LMA in pediatric patients are limited. Endigeri et al. [
16] reported an OPLP of Blockbuster
TM LMA of 33.7 ± 1.8 cmH
2O in adult patients, which is higher than observed in our study. Though a higher OPLP doesn't always guarantee an appropriate placement, it is commonly used objective test to guide correct placement. A higher OPLP suggests proper placement of device around perilaryngeal structure and ability of the device to sustain leak during positive pressure ventilation. Though in our study a statistically significant difference was observed between two devices, with I-gel
TM reported to have higher seal pressure, but clinically this difference was not very significant as if SGA is achieving an OPLP more than 20 cmH
2O, it can sustain leak during spontaneous or controlled ventilation in pediatric patients. There are plenty of studies in I-gel
TM in pediatric patients and I-gel
TM is considered to be a prototype second generation LMA but Blockbuster
TM LMA is newly introduced with limited studies available on its use in children. So, our study finding support the use of Blockbuster
TM LMA for pediatric airway management, however further randomized controlled trial are required to confirm and support our study findings. We inflated the Blockbuster
TM LMA as per manufacturer specification but further study of inflation pressures and positioning may help optimize the Blockbuster
TM LMA.
There was no case of failed insertion of the LMA in either group, with airways secured in 97% and 90% of the first attempts in the I-gel
TM and Blockbuster
TM LMA groups respectively. Studies have reported a greater than 95% first-attempt success rate for I-gel
TM [
17]. Ease of insertion was comparable for both I-gel
TM and Blockbuster
TM LMA with 100% of both groups having “easy” and “very easy” insertion. Total insertion time was comparable between I-gel
TM and Blockbuster
TM LMA at 15.51 ± 1.62 and 15.92 ± 3.02 s respectively. All insertions were performed in < 30 s which is acceptable given the time required to secure an airway. Both I-gel
TM and the Blockbuster
TM LMA had a sleek and streamlined design that could be deployed quickly, even considering the anatomical challenges presented by pediatric airways. Previous studies have found that I-gel
TM took a longer time to insert and this was attributed to I-gel
TM straight shape which showed frequent displacements. It has also been shown that the I-gel
TM requires consistent downward mechanical pressure to stay in place with close contact with the glottis. We had no such difficulties in our study and were able to promptly fix I-gel
TM in place. The Blockbuster
TM LMA with its inflatable cuff and the angled tube, did not cause any difficulties worth mentioning during insertion.
All hemodynamic variables were comparable in both groups with no significant change in parameters at different intervals in the study. Similarly, the incidence of postoperative complications was zero in I-gelTM and one incidence of blood stains in BlockbusterTM LMA. All insertions were performed by trained individuals and therefore the optimal safety seen with both SGA devices was to be expected.
Limitations of our study include enrolling subjects with normal airways with no prior anatomical pathology. Second, we did not evaluate the additional features of both LMAs such as gastric channels and the ability to be used as a channel for intubation. Third, we have not confirmed the fiberoptic position of any of the LMA after insertion and we have not evaluated the inflation cuff pressure in the BlockbusterTM LMA.
To conclude, the I-gelTM delivered significantly higher OPLP than BlockbusterTM LMA, otherwise, both LMAs are comparable in terms of performance. Both I-gelTM and BlockbusterTM LMA are appropriate devices for positive pressure ventilation in pediatric patients undergoing short surgical procedures under general anesthesia with minimal pharyngolaryngeal morbidity.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download