Abstract
Background
The correlation between acute coronavirus disease 2019 (COVID-19) and subacute thyroiditis (SAT) has not been clearly investigated in “long COVID” patients. We aimed to investigate the incidence of SAT during convalescence and after the acute phase of COVID-19, comparing with that of the general population.
Methods
Data from a total of 422,779 COVID-19 patients and a control group of 2,113,895 individuals were analyzed. The index date was defined as the date 3 months after confirmation of COVID-19. The incidence rate (IR) of SAT and hazard ratios (HRs) were calculated per 100,000 persons. Subgroup analysis included analysis of HRs 90–179 and 180 days post-COVID-19 diagnosis; and additional analysis was conducted according to hospitalization status, sex, and age group.
Results
The IR of SAT was 17.28 per 100,000 persons (95% confidence interval [CI], 12.56 to 23.20) in the COVID-19 group and 8.63 (95% CI, 6.37 to 11.45) in the control group. The HR of COVID-19 patients was 1.76 (95% CI, 1.01 to 3.06; P=0.045). The HR of SAT was 1.39 (95% CI, 0.82 to 2.34; P=0.220) up to 6 months after the index date and 2.30 (95% CI, 1.60 to 3.30; P<0.001) beyond 6 months. The HR for SAT among COVID-19 patients was 2.00 (95% CI, 1.41 to 2.83) in hospitalized patients and 1.76 (95% CI, 1.01 to 3.06) in non-hospitalized patients compared to the control group. The IR of SAT was 27.09 (95% CI, 20.04 to 35.82) for females and 6.47 (95% CI, 3.34 to 11.30) for males. In the 19 to 64 age group, the IR of SAT was 18.19 (95% CI, 13.70 to 23.67), while the IR was 9.18 (95% CI, 7.72 to 10.84) in the 65 to 69 age group.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been impacting global health since first scientifically described in December 2019 and was classified as a pandemic by the World Health Organization (WHO) in March 2020. However, as coronavirus disease 2019 (COVID-19) has transitioned to an endemic disease in some regions, its impact on global health is constantly evolving [1].
SARS-CoV-2 infects the host’s cells through the angiotensin-converting enzyme 2 (ACE2) receptor by binding of the SARS-CoV-2 spike or S protein to the ACE2 receptor [2,3]. Viral RNA has been found in serum, urine, and fecal samples of infected COVID-19 patients, suggesting that SARS-CoV-2 could have a systemic effect on multiple organs with ACE2 expression and could result in multi-systemic failure [4]. In particular, high ACE expression levels have been detected in the thyroid gland [5,6]. Triiodothyronine and thyroxine are correlated with serum ACE2 levels and are crucial for adaptive immune response [7]. Several hypotheses that contribute to the increased risk of inflammatory thyroidal disease in COVID-19 patients have been suggested. One is the destruction of follicular cells by autoimmune and/or viral thyroiditis [8]. A second is the initiation of a cytokine storm from the increased production of proinflammatory cytokine such as interleukin-6 and tumor necrosis factor causing thyroid hyperfunction [9]. Although the correlation between subacute thyroiditis (SAT) and viral infection has been well-established, the mechanism, pathogenesis, and consequences of thyroid involvement in COVID-19 patients are not clearly understood. Moreover, previous studies have only focused on new-onset SAT during the acute phase of COVID-19 [7,9-11].
Approximately 10% of COVID-19 patients have reported prolonged symptoms beyond 4 weeks after diagnosis, known as “long COVID” [12,13] or “post-COVID conditions” according to the Centers for Disease Control and Prevention [14]. Previous research has reported that 1.9% of patients with normal thyroid function progressed to thyroiditis during convalescence [15]. In terms of long COVID, considering the possibility of late-onset thyroiditis after a longer period following diagnosis of COVID-19 is important. Long COVID was defined as the persistence of symptoms and signs after 12 weeks following COVID-19 diagnosis by the Korea Disease Control and Prevention and the Korean Society of Infectious Diseases [16]. Therefore, we aimed to establish the nationwide incidence of SAT during convalescence and after the acute phase of COVID-19 and to compare this incidence to that of the general population, thereby determining whether post-COVID-19 SAT presents a public health burden.
This population-based retrospective and cross-sectional study used data from the Health Insurance Review and Assessment (HIRA) claims database, which contains information on individuals who underwent SARS-CoV-2 real-time reverse transcriptase polymerase chain reaction testing of nasal or pharyngeal swabs in accordance with WHO guidelines. The participants with COVID-19 infection between January 2020 and September 2021 in the National Health Insurance Service and Korea Disease Control and Prevention Agency databases were included. The HIRA database is a comprehensive healthcare dataset that is managed by the Korean government and encompasses nearly 100% of the country’s population. The database includes information on the beneficiaries’ demographic characteristics, healthcare utilization history based on the International Classification of Diseases 10th Revision (ICD-10), and hospitalization and outpatient care prescriptions.
COVID-19 patients were defined by ICD-10 codes U18 (provisional assignment of new diseases of uncertain etiology or emergency use), U07.1 (COVID-19), B34.2 (coronavirus infection, unspecified site), and B97.2 (coronavirus as the cause of diseases classified to other chapters). A total of 432,795 patients with COVID-19, ranging in age from 19 to 89 years, were enrolled in the study. Out of these, 25,386 subjects were excluded based on the following criteria: patients who received a steroid prescription for SAT as a diagnosis within the past year before the index date, subjects who died before the index date, subjects who underwent thyroidectomy (as indicated by HIRA procedure codes P4561, P4552, P4554, P4551, and P4553) and individual who had a presence of autoimmune thyroiditis (ICD-10 code, E06.3). The index date was defined as the date 3 months after confirmation of COVID-19. We matched COVID-19 patients with a control group of 2,037,135 individuals in a 1:5 ratio based on age and sex. Ultimately, data from a total of 407,427 COVID-19 patients and 2,037,135 control subjects were analyzed, and these subjects were followed from baseline to April 30, 2022. This study complied with the ethical standards of the Declaration of Helsinki (IRB approval No. 2022-081). The protocol using secondary data received approval from the Eunpyeong St. Mary’s Hospital Institutional Review Board of Catholic Medical Center, Catholic University of Korea (IRB approval No. PC20ZASI0091). The requirement for written informed consent was waived due to the use of previously collected and anonymized data.
In our study, we evaluated the incidence of SAT, which was defined as the presence of ICD-10 code E061 (SAT) at least one time during the study period. To identify cases of SAT, we selected patients who had both the E061 code and a prescription for corticosteroids concurrently.
Baseline characteristics are presented as the mean±standard deviation, median (interquartile range), or number (%). The incidence rate (IR) of SAT was calculated as the number of events divided by the total follow-up duration (person-years) and standardized the rate per 100,000 person-years, and the 95% confidence interval (CI) and P value were calculated using a Poisson approximation. The risk ratio of COVID-19 patients is presented as hazard ratios (HRs) with 95% CIs using Cox proportional hazards models for matched data compared to the control group. In subanalysis, the HR for COVID-19 was evaluated according to hospitalized patients and non-hospitalized status, gender and age with the control group as reference. All tests were two-sided and a P value <0.05 was considered statistically significant. All statistical analyses were performed using the R program version 4.1.1 (R Development Core Team, Vienna, Austria).
A total of 244,562 subjects were analyzed. The mean age of all study subjects during the follow-up period was 46.3±17.4 years. Of the analyzed subject data, 50.4% of subjects were male. The mean follow-up time from the index date after COVID-19 was 10.8±5.6 months. Table 1 presents the baseline characteristics of COVID-19 patients and the control group after 1:5 matching.
During the follow-up period, the IR of SAT demonstrated different patterns in COVID-19 patients compared to the control group. A total of 220 SAT events occurred in the entire cohort during 2,205,713 person-years of follow-up and resulted in an IR of 9.97 (95% CI, 8.70 to 11.38), The mean of age was 46.5 years. The events of SAT were 87.6% in women and 12.4% in men (Table 2).
In COVID-19 patients, 61 cases of SAT were reported during 366,346 person-years of follow-up, resulting in an IR of 16.65 (95% CI, 12.74 to 2.59). The IR in the general population was 9.97 (95% CI, 8.70 to 11.38). COVID-19 patients had a significantly higher IR compared to the control group with a HR of 1.93 (95% CI, 1.43 to 2.59; P<0.01).
The cumulative incidence of SAT was higher in the COVID-19 group compared to the control group over a 2-year period. The HR for COVID-19 was 1.39 (95% CI, 0.82 to 2.34; P=0.220) at 90 to 179 days after diagnosis and increased to 2.30 (95% CI, 1.60 to 3.30; P<0.001) after 180 days compared to the control group. During the period of 560 days following COVID-19 diagnosis, the IR of SAT was found to be 5.05 (95% CI, 1.89 to 13.45; P=0.001) (Fig. 1).
Table 3 shows the incidence of SAT according to severity of COVID-19, sex, and age. In subanalysis, hospitalized patients with COVID-19 had an IR for SAT of 17.28 (95% CI, 12.56 to 23.20), and those without COVID-19 had IR of 15.21 (95% CI, 8.86 to 24.35). The HR in hospitalized patients for COVID-19 was 2.00 (95% CI, 1.41 to 2.83; P<0.001). In the outpatient clinic, the HR with 95% CI for COVID-19 was 1.76 (95% CI, 1.01 to 3.06; P=0.153). The IR of SAT was 27.09 (95% CI, 20.04 to 35.82) for females and 6.47 (95% CI, 3.34 to 11.30) for males. However, the HR for SAT in females was 1.73 (95% CI, 1.25 to 2.39; P=0.001) and 3.54 (95% CI, 1.69 to 7.42; P=0.001) in males. The IR of SAT was 18.19 (95% CI, 13.70 to 23.67) in the 19 to 64 age group, while the IR was 9.39 (95% CI, 3.45 to 20.43) in the 65 to 89 age group. The HR of SAT was 1.98 (95% CI, 1.45 to 2.71; P<0.01) in younger patients and 1.52 (95% CI, 0.61 to 3.79; P=0.368) in the older group.
COVID-19 has been associated with a spectrum of prolonged health sequelae, including respiratory and cardiovascular domains [17-19]. Previous studies reported that long COVID affects long-term morbidity and mortality [20,21]. Therefore, our study aimed to investigate the association between COVID-19 and the incidence of SAT during the post-acute phase for COVID-19. Our results showed a significantly higher IR of SAT in COVID-19 patients compared to the control group, with a HR of 1.93. The IR of SAT showed no significant difference during the first 6 months following the index date. However, from 6 months onward, the IR increased two-fold. These results suggest the potential long-term impact of COVID-19 on thyroid function and long-term surveillance of COVID-19-related health outcomes is required.
The IR of SAT during the pandemic era has been reported to range from 7.3 to 11.4 per 100,000 person-years in various studies [22-24]. Our study found that the IR of SAT in the general population was 8.64. This is consistent with previous research that reported an IR of 8.3 [22]. In COVID-19 infection, the IR of SAT was 16.82, 1.93-fold higher than in the control group. This finding is consistent with previous studies that also reported an increased risk of SAT in COVID-19 infection. Muller et al. [25] found a higher prevalence of SAT in hospitalized COVID-19 patients compared to non-COVID-19 patient. However, this study only included hospitalized patients and did not include a control group from the general population. In contrast, our study included a control group from the general population. A recent systematic review and meta-analysis found that COVID-19 infection was associated with a significantly increased risk of SAT, with a pooled odds ratio of 2.90 (95% CI, 2.15 to 3.92) [26]. Lui et al. [27] reported a lower prevalence of SAT (13.9%) in patients with mild COVID-19, which is similar to that observed in non-COVID-19 controls. However, this study only included patients with mild COVID-19, and thus the prevalence of SAT in severe COVID-19 cases remains unclear. These studies had small sample sizes.
The IR for SAT in females with COVID-19 was 27.09 per 100,000 person-years and the IR in females without COVID-19, with same age distribution as COVID-19 patients, was 15.61 per 100,000 person-years. This showed that females had 11.44 more cases per 100,000 person-years. This figure in males with COVID-19 was 3.54 per 100,000 person-years. This trend is consistent with previous studies that reported a higher prevalence of SAT in females (75% to 80%) [28,29]. A recent study suggests that sex differences in immune responses and the presence of multiple immune-related genes on the X-chromosome may explain the higher prevalence of immune diseases in females [30]. However, our findings indicated that male gender is more correlated to developing SAT after COVID-19 infection. Thus, the biological mechanism underlying the observed sex differences in the incidence of SAT remains unclear. Additionally, the HR of SAT was 1.98 in younger patients (19 to 64 years) and 1.52 in elderly patients (65 to 89 years). This finding could be explained by age-related changes in the immune system, resulting in a decreased immune response to infections in the elderly population. In contrast, a more robust immune response to infections is preserved in younger patients and it leads to increase the risk of SAT following COVID-19 [31]. Another plausible mechanism for the higher incidence of SAT in young age could be attributed to the higher expression of ACE2 [32]. The increased expression of ACE2 in younger age groups may render the thyroid gland more susceptible to viral entry. This heightened susceptibility could potentially lead to viral infection, subsequent inflammation, and the development of SAT. However, age-related differences in SAT incidence following COVID-19 has not been clearly documented.
Our study found the incidence of SAT was approximately twofold higher in the COVID-19 group after 6 months following infection compared to the control group. However, there was no significant difference in the incidence between the COVID-19 group and the control group within the period of 90 to 179 days. Regarding the relationship between SAT and long COVID, there is limited research. A majority of previous studies has focused on the acute phase of COVID-19 within 8 to 12 weeks [10,11,18,23,27]. A recent study from Hong Kong reported that there were no significant differences between patients with and without long COVID at 6 months. This included consideration of disease COVID-19 severity at baseline [33]. However, the size of the severe COVID-19 group in that study was relatively small. The underlying mechanisms of SAT in long COVID patients are not yet fully established. Previous studies have reported rates of up to 33% for thyroid dysfunction and 20% for autoimmune thyroiditis in patients treated with interferon beta-1b [34,35]. One plausible mechanism is that the treatment using interferon therapy in COVID-19 patients may directly affect the thyroid gland and on iodine organification, leading to inflammation and subsequent damage to the thyroid cells during the post-acute phase [36]. Another possibility is that chronic exposure to interferon may trigger autoimmune reactions or disrupt the normal functioning of the immune system, potentially leading to thyroid-related complications. Another possibility is that unwanted autoimmune reaction [37]. These mechanisms may contribute to the development of SAT in long COVID patients. However, we encountered challenges in obtaining data for the study period due to the delayed availability of billing data retrieval.
To the best of our knowledge, this is the first study to investigate the incidence of SAT in long COVID cases. This study is based on a large sample size from a nationally representative database. Thus the results should reflect clinical practice experience. Second, the use of a 1:5 matching strategy of COVID-19 patients to the general population enhances the reliability of our results and minimize selection bias. Despite these strengths, our study had several limitations. First, the database of claims data we used did not provide detailed clinical information on COVID-19 patients, such as disease severity, biochemistry such as thyroid function and thyroid antibody test results, treatment of COVID-19 or vaccination. Therefore, the causal relationship between COVID-19 and SAT could not be assessed due to the study design. Second, the accuracy of our findings is reliant on the quality and completeness of the HIRA database, which may contain inaccuracies or missing information. Finally, we cannot completely rule out the possibility of misclassification bias, as the identification of SAT was based on ICD-10 codes. The operational definition of SAT did not include the erythrocyte sedimentation rate (ESR), which is crucial maker for SAT. By not incorporating ESR into definition, some cases could be missed or misclassified. However, we took measures to ensure consistency in conditions for both the patient and control groups to enhance the reliability of our study.
In conclusion, our findings showed that SAT could be a potential long-term complication of COVID-19. These findings suggest the importance of long-term surveillance of COVID-19 patients, especially focusing on monitoring for signs of thyroid dysfunction. Further research is needed to investigate the underlying mechanisms and to prove the causality between long COVID-19 and SAT.
ACKNOWLEDGMENTS
This research was supported by a grant of the Big Data Research Project through the Korean Endocrine Society.
REFERENCES
1. World Health Organization. COVID-19 weekly epidemiological update, edition 115, 26 October 2022 [Internet]. Geneva: WHO;2022. [cited 2023 Jul 11]. Available from: https://apps.who.int/iris/handle/10665/363853.
2. Ziegler CG, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020; 181:1016–35.
3. Chen W, Tian Y, Li Z, Zhu J, Wei T, Lei J. Potential interaction between SARS-CoV-2 and thyroid: a review. Endocrinology. 2021; 162:bqab004.
4. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020; 323:1843–4.
5. Lazartigues E, Qadir MM, Mauvais-Jarvis F. Endocrine significance of SARS-CoV-2’s reliance on ACE2. Endocrinology. 2020; 161:bqaa108.
6. Duntas LH, Jonklaas J. COVID-19 and thyroid diseases: a bidirectional impact. J Endocr Soc. 2021; 5:bvab076.
7. Zhang Y, Lin F, Tu W, Zhang J, Choudhry AA, Ahmed O, et al. Thyroid dysfunction may be associated with poor outcomes in patients with COVID-19. Mol Cell Endocrinol. 2021; 521:111097.
8. Boguslawska J, Godlewska M, Gajda E, Piekielko-Witkowska A. Cellular and molecular basis of thyroid autoimmunity. Eur Thyroid J. 2022; 11:e210024.
9. Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol. 2020; 183:381–7.
10. Giovanella L, Ruggeri RM, Ovcaricek PP, Campenni A, Treglia G, Deandreis D. Prevalence of thyroid dysfunction in patients with COVID-19: a systematic review. Clin Transl Imaging. 2021; 9:233–40.
11. Naguib R. Potential relationships between COVID-19 and the thyroid gland: an update. J Int Med Res. 2022; 50:3000605221082898.
12. Ladds E, Rushforth A, Wieringa S, Taylor S, Rayner C, Husain L, et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res. 2020; 20:1144.
13. Yong SJ, Halim A, Halim M, Liu S, Aljeldah M, Al Shammari BR, et al. Inflammatory and vascular biomarkers in post-COVID-19 syndrome: a systematic review and meta-analysis of over 20 biomarkers. Rev Med Virol. 2023; 33:e2424.
14. Centers for Disease Control and Prevention. Post-COVID conditions: CDC science [Internet]. Atlanta: CDC;2022. [cited 2023 Jul 11]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-science.html.
15. Lui DT, Lee CH, Chow WS, Lee AC, Tam AR, Fong CH, et al. Insights from a prospective follow-up of thyroid function and autoimmunity among COVID-19 survivors. Endocrinol Metab (Seoul). 2021; 36:582–9.
16. Korea Disease Control and Prevention Agency. Long COVID [Internet]. Cheongju: KDCA;2023. [cited 2023 Jul 11]. Available from: https://ncv.kdca.go.kr/hcp/page.do?mid=0102.
17. Amenta EM, Spallone A, Rodriguez-Barradas MC, El Sahly HM, Atmar RL, Kulkarni PA. Postacute COVID-19: an overview and approach to classification. Open Forum Infect Dis. 2020; 7:ofaa509.
18. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate COVID-19. N Engl J Med. 2020; 383:1757–66.
19. Yin T, Li Y, Ying Y, Luo Z. Prevalence of comorbidity in Chinese patients with COVID-19: systematic review and meta-analysis of risk factors. BMC Infect Dis. 2021; 21:200.
20. Sunada N, Honda H, Nakano Y, Yamamoto K, Tokumasu K, Sakurada Y, et al. Hormonal trends in patients suffering from long COVID symptoms. Endocr J. 2022; 69:1173–81.
21. Boaventura P, Macedo S, Ribeiro F, Jaconiano S, Soares P. Post-COVID-19 condition: where are we now? Life (Basel). 2022; 12:517.
22. Ahn HY, Choi HS, Ha S, Cho SW. Incidence of subacute thyroiditis during the COVID-19 pandemic in South Korea using the National Health Insurance Service Database. Thyroid. 2022; 32:1299–306.
23. Domin R, Szczepanek-Parulska E, Dadej D, Ruchala M. Subacute thyroiditis: literature overview and COVID-19. J Med Sci. 2020; 89:e472.
24. Brancatella A, Viola N, Santini F, Latrofa F. COVID-induced thyroid autoimmunity. Best Pract Res Clin Endocrinol Metab. 2023; 37:101742.
25. Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, Muscatello A, et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020; 8:739–41.
26. Darvishi M, Nazer MR, Shahali H, Nouri M. Association of thyroid dysfunction and COVID-19: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2022; 13:947594.
27. Lui DT, Fung MM, Chiu KW, Lee CH, Chow WS, Lee AC, et al. Higher SARS-CoV-2 viral loads correlated with smaller thyroid volumes on ultrasound among male COVID-19 survivors. Endocrine. 2021; 74:205–14.
28. Stasiak M, Michalak R, Stasiak B, Lewinski A. Clinical characteristics of subacute thyroiditis is different than it used to be: current state based on 15 years own material. Neuro Endocrinol Lett. 2019; 39:489–95.
29. Stasiak M, Lewinski A. New aspects in the pathogenesis and management of subacute thyroiditis. Rev Endocr Metab Disord. 2021; 22:1027–39.
30. Angum F, Khan T, Kaler J, Siddiqui L, Hussain A. The prevalence of autoimmune disorders in women: a narrative review. Cureus. 2020; 12:e8094.
31. Gu J, Yin J, Zhang M, Li J, Wu Y, Chen J, et al. Study on the clinical significance of ACE2 and its age-related expression. J Inflamm Res. 2021; 14:2873–82.
32. Lui DT, Lee CH, Chow WS, Lee AC, Tam AR, Fong CH, et al. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab. 2021; 106:e926–35.
33. Lui DT, Tsoi KH, Lee CH, Cheung CY, Fong CH, Lee AC, et al. A prospective follow-up on thyroid function, thyroid autoimmunity and long COVID among 250 COVID-19 survivors. Endocrine. 2023; 80:380–91.
34. Monzani F, Caraccio N, Casolaro A, Lombardo F, Moscato G, Murri L, et al. Long-term interferon beta-1b therapy for MS: is routine thyroid assessment always useful? Neurology. 2000; 55:549–52.
35. Kreisler A, de Seze J, Stojkovic T, Delisse B, Combelles M, Verier A, et al. Multiple sclerosis, interferon beta and clinical thyroid dysfunction. Acta Neurol Scand. 2003; 107:154–7.
36. Frisullo G, Calabrese M, Tortorella C, Paolicelli D, Ragonese P, Annovazzi P, et al. Thyroid autoimmunity and dysfunction in multiple sclerosis patients during long-term treatment with interferon beta or glatiramer acetate: an Italian multicenter study. Mult Scler. 2014; 20:1265–8.
37. Watad A, David P, Brown S, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants and thyroid autoimmunity. Front Endocrinol (Lausanne). 2017; 7:150.
Fig. 1.
The cumulative incidence of subacute thyroiditis in coronavirus disease 2019 (COVID-19) patients and general population.
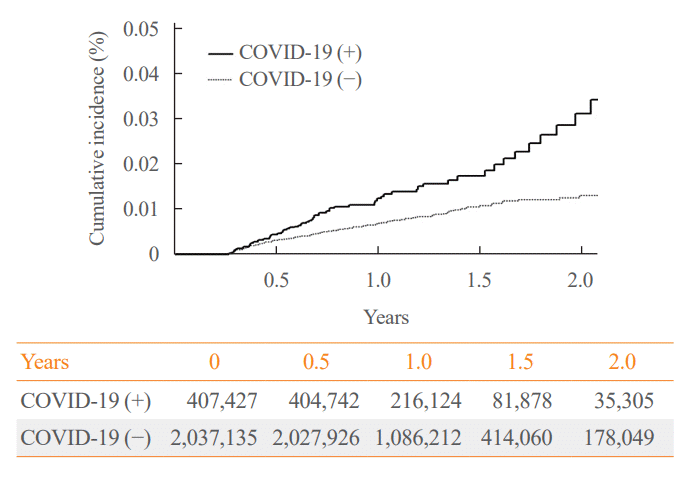
Table 1.
Baseline Characteristics of Subject with COVID-19 and without COVID-19
Table 2.
Incidence of Subacute Thyroiditis according to COVID-19 Infection
Table 3.
The Incidence of Subacute Thyroiditis according to Parameters




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download