1. Joseph P, Leong D, McKee M, Anand SS, Schwalm JD, Teo K, et al. Reducing the global burden of cardiovascular disease, Part 1: The epidemiology and risk factors. Circ Res. 2017; 121(6):677–694. PMID:
28860318.
2. Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017; 70(1):1–25. PMID:
28527533.
3. Mehra R. Global public health problem of sudden cardiac death. J Electrocardiol. 2007; 40(6):Suppl. S118–S122. PMID:
17993308.
4. Fuller JH, Stevens LK, Wang SL. Risk factors for cardiovascular mortality and morbidity: the WHO Mutinational Study of Vascular Disease in Diabetes. Diabetologia. 2001; 44(Suppl 2):S54–S64. PMID:
11587051.
5. Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998; 97(18):1837–1847. PMID:
9603539.
6. Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995; 274(17):1363–1367. PMID:
7563561.
7. Whang W, Manson JE, Hu FB, Chae CU, Rexrode KM, Willett WC, et al. Physical exertion, exercise, and sudden cardiac death in women. JAMA. 2006; 295(12):1399–1403. PMID:
16551711.
8. Sheerah HA, Eshak ES, Cui R, Imano H, Iso H, Tamakoshi A, et al. Relationship between dietary vitamin D and deaths from stroke and coronary heart disease: the Japan Collaborative Cohort Study. Stroke. 2018; 49(2):454–457. PMID:
29311267.
9. Drechsler C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J. 2010; 31(18):2253–2261. PMID:
20688781.
10. Judd SE, Tangpricha V. Vitamin D deficiency and risk for cardiovascular disease. Am J Med Sci. 2009; 338(1):40–44. PMID:
19593102.
11. Vaidya A, Williams JS. The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism. 2012; 61(4):450–458. PMID:
22075270.
12. Kassi E, Adamopoulos C, Basdra EK, Papavassiliou AG. Role of vitamin D in atherosclerosis. Circulation. 2013; 128(23):2517–2531. PMID:
24297817.
13. Calton EK, Keane KN, Newsholme P, Soares MJ. The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One. 2015; 10(11):e0141770. PMID:
26528817.
14. Gouni-Berthold I, Krone W, Berthold HK. Vitamin D and cardiovascular disease. Curr Vasc Pharmacol. 2009; 7(3):414–422. PMID:
19601865.
15. Polly P, Tan TC. The role of vitamin D in skeletal and cardiac muscle function. Front Physiol. 2014; 5:145. PMID:
24782788.
16. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6(7):e1000097. PMID:
19621072.
17. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7(3):177–188. PMID:
3802833.
18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327(7414):557–560. PMID:
12958120.
19. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315(7109):629–634. PMID:
9310563.
20. Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med. 2001; 20(4):641–654. PMID:
11223905.
21. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992; 135(11):1301–1309. PMID:
1626547.
22. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized doseresponse data. Stata J. 2006; 6(1):46–57.
23. Yu C, Cao Q, Chen P, Yang S, Deng M, Wang Y, et al. An updated dose-response meta-analysis of coffee consumption and liver cancer risk. Sci Rep. 2016; 6(1):37488. PMID:
27910873.
24. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington, D.C., USA: National Academy Press;2010.
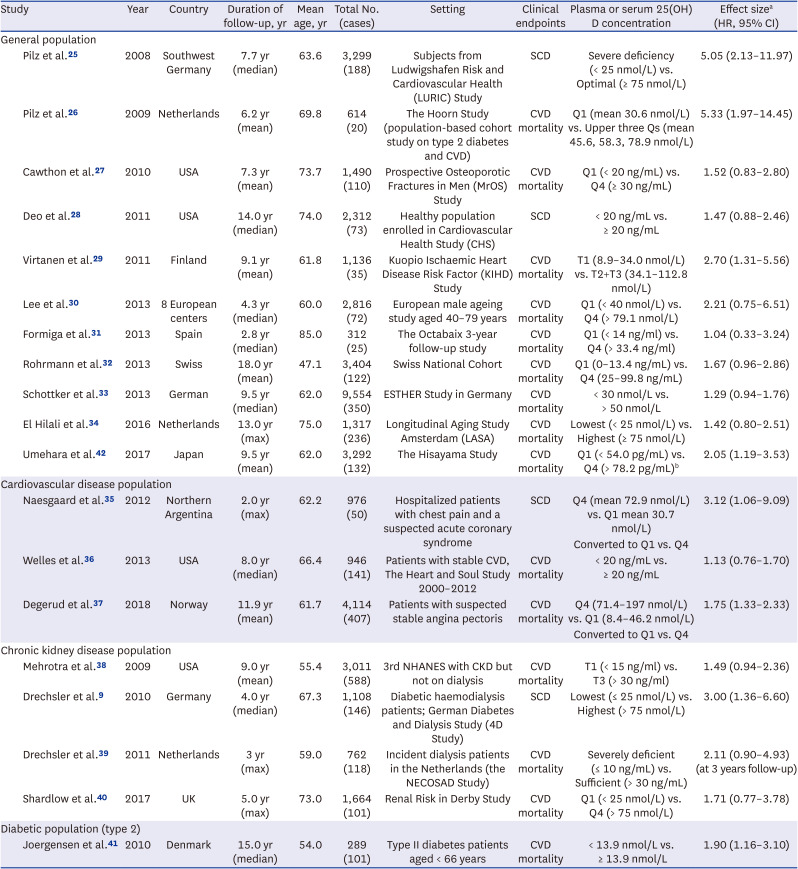
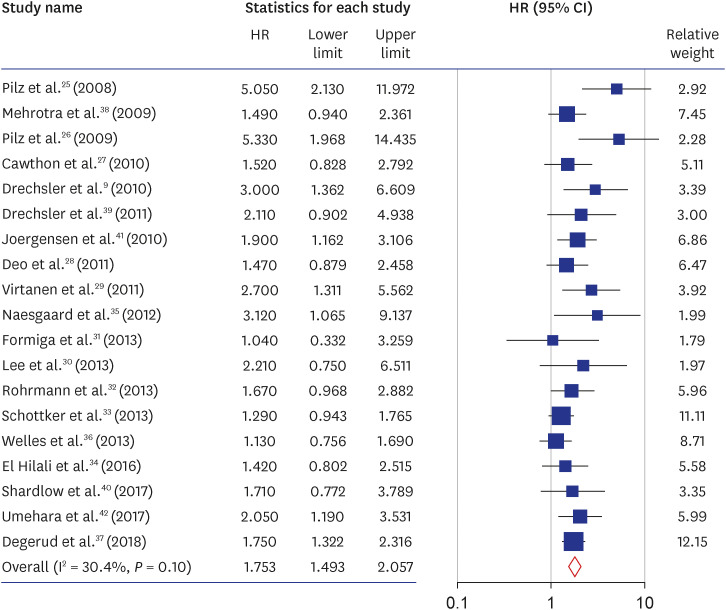
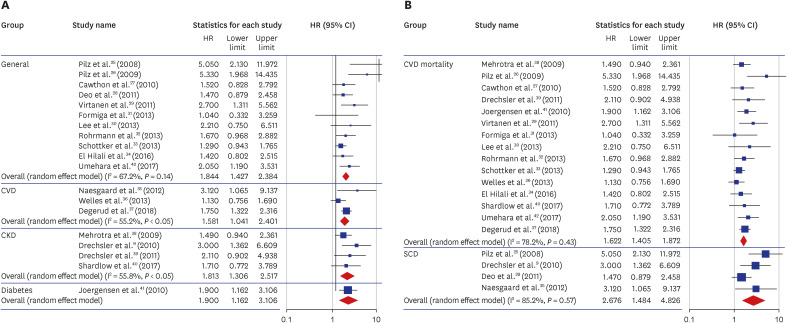
25. Pilz S, März W, Wellnitz B, Seelhorst U, Fahrleitner-Pammer A, Dimai HP, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008; 93(10):3927–3935. PMID:
18682515.
26. Pilz S, Dobnig H, Nijpels G, Heine RJ, Stehouwer CD, Snijder MB, et al. Vitamin D and mortality in older men and women. Clin Endocrinol (Oxf). 2009; 71(5):666–672. PMID:
19226272.
27. Cawthon PM, Parimi N, Barrett-Connor E, Laughlin GA, Ensrud KE, Hoffman AR, et al. Serum 25-hydroxyvitamin D, parathyroid hormone, and mortality in older men. J Clin Endocrinol Metab. 2010; 95(10):4625–4634. PMID:
20631024.
28. Deo R, Katz R, Shlipak MG, Sotoodehnia N, Psaty BM, Sarnak MJ, et al. Vitamin D, parathyroid hormone, and sudden cardiac death: results from the Cardiovascular Health Study. Hypertension. 2011; 58(6):1021–1028. PMID:
22068871.
29. Virtanen JK, Nurmi T, Voutilainen S, Mursu J, Tuomainen TP. Association of serum 25-hydroxyvitamin D with the risk of death in a general older population in Finland. Eur J Nutr. 2011; 50(5):305–312. PMID:
20976461.
30. Lee DM, Vanderschueren D, Boonen S, O’Neill TW, Pendleton N, Pye SR, et al. Association of 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and parathyroid hormone with mortality among middle-aged and older European men. Age Ageing. 2014; 43(4):528–535. PMID:
24375224.
31. Formiga F, Ferrer A, Megido MJ, Boix L, Contra A, Pujol R. Octabaix study members. Low serum vitamin D is not associated with an increase in mortality in oldest old subjects: the Octabaix three-year follow-up study. Gerontology. 2014; 60(1):10–15. PMID:
23689215.
32. Rohrmann S, Braun J, Bopp M, Faeh D. Swiss National Cohort (SNC). Inverse association between circulating vitamin D and mortality--dependent on sex and cause of death? Nutr Metab Cardiovasc Dis. 2013; 23(10):960–966. PMID:
24095147.
33. Schöttker B, Haug U, Schomburg L, Köhrle J, Perna L, Müller H, et al. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am J Clin Nutr. 2013; 97(4):782–793. PMID:
23446902.
34. El Hilali J, de Koning EJ, van Ballegooijen AJ, Lips P, Sohl E, van Marwijk HW, et al. Vitamin D, PTH and the risk of overall and disease-specific mortality: results of the Longitudinal Aging Study Amsterdam. J Steroid Biochem Mol Biol. 2016; 164:386–394. PMID:
26678328.
35. Naesgaard PA, León De La Fuente RA, Nilsen ST, Woie L, Aarsland T, Brede C, et al. Serum 25(OH)D is a 2-year predictor of all-cause mortality, cardiac death and sudden cardiac death in chest pain patients from Northern Argentina. PLoS One. 2012; 7(9):e43228. PMID:
22970121.
36. Welles CC, Whooley MA, Karumanchi SA, Hod T, Thadhani R, Berg AH, et al. Vitamin D deficiency and cardiovascular events in patients with coronary heart disease: data from the Heart and Soul Study. Am J Epidemiol. 2014; 179(11):1279–1287. PMID:
24699783.
37. Degerud E, Nygård O, de Vogel S, Hoff R, Svingen GF, Pedersen ER, et al. Plasma 25-hydroxyvitamin D and mortality in patients with suspected stable angina pectoris. J Clin Endocrinol Metab. 2018; 103(3):1161–1170. PMID:
29325121.
38. Mehrotra R, Kermah DA, Salusky IB, Wolf MS, Thadhani RI, Chiu YW, et al. Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int. 2009; 76(9):977–983. PMID:
19657329.
39. Drechsler C, Verduijn M, Pilz S, Dekker FW, Krediet RT, Ritz E, et al. Vitamin D status and clinical outcomes in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant. 2011; 26(3):1024–1032. PMID:
20947538.
40. Shardlow A, McIntyre NJ, Fluck RJ, McIntyre CW, Taal MW. Associations of fibroblast growth factor 23, vitamin D and parathyroid hormone with 5-year outcomes in a prospective primary care cohort of people with chronic kidney disease stage 3. BMJ Open. 2017; 7(8):e016528.
41. Joergensen C, Gall MA, Schmedes A, Tarnow L, Parving HH, Rossing P. Vitamin D levels and mortality in type 2 diabetes. Diabetes Care. 2010; 33(10):2238–2243. PMID:
20606205.
42. Umehara K, Mukai N, Hata J, Hirakawa Y, Ohara T, Yoshida D, et al. Association between serum vitamin D and all-cause and cause-specific death in a general Japanese population - The Hisayama Study. Circ J. 2017; 81(9):1315–1321. PMID:
28428487.
43. Al Mheid I, Patel RS, Tangpricha V, Quyyumi AA. Vitamin D and cardiovascular disease: is the evidence solid? Eur Heart J. 2013; 34(48):3691–3698. PMID:
23751422.
44. Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008; 168(12):1340–1349. PMID:
18574092.
45. Choudhury S, Bae S, Ke Q, Lee JY, Singh SS, St-Arnaud R, et al. Abnormal calcium handling and exaggerated cardiac dysfunction in mice with defective vitamin D signaling. PLoS One. 2014; 9(9):e108382. PMID:
25268137.
46. Fanari Z, Hammami S, Hammami MB, Hammami S, Abdellatif A. Vitamin D deficiency plays an important role in cardiac disease and affects patient outcome: still a myth or a fact that needs exploration? J Saudi Heart Assoc. 2015; 27(4):264–271. PMID:
26557744.
47. Papapetrou PD. The interrelationship of serum 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D in pregnancy at term: a meta-analysis. Hormones (Athens). 2010; 9(2):136–144. PMID:
20687397.
48. Cashman KD, van den Heuvel EG, Schoemaker RJ, Prévéraud DP, Macdonald HM, Arcot J. 25-Hydroxyvitamin D as a biomarker of vitamin D status and its modeling to inform strategies for prevention of vitamin D deficiency within the population. Adv Nutr. 2017; 8(6):947–957. PMID:
29141976.
49. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006; 116(8):2062–2072. PMID:
16886050.
50. Lhamo Y, Chugh PK, Gautam SR, Tripathi CD. Epidemic of vitamin D deficiency and its management: awareness among Indian medical undergraduates. J Environ Public Health. 2017; 2017:2517207. PMID:
28473860.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download