Abstract
Background
Methods
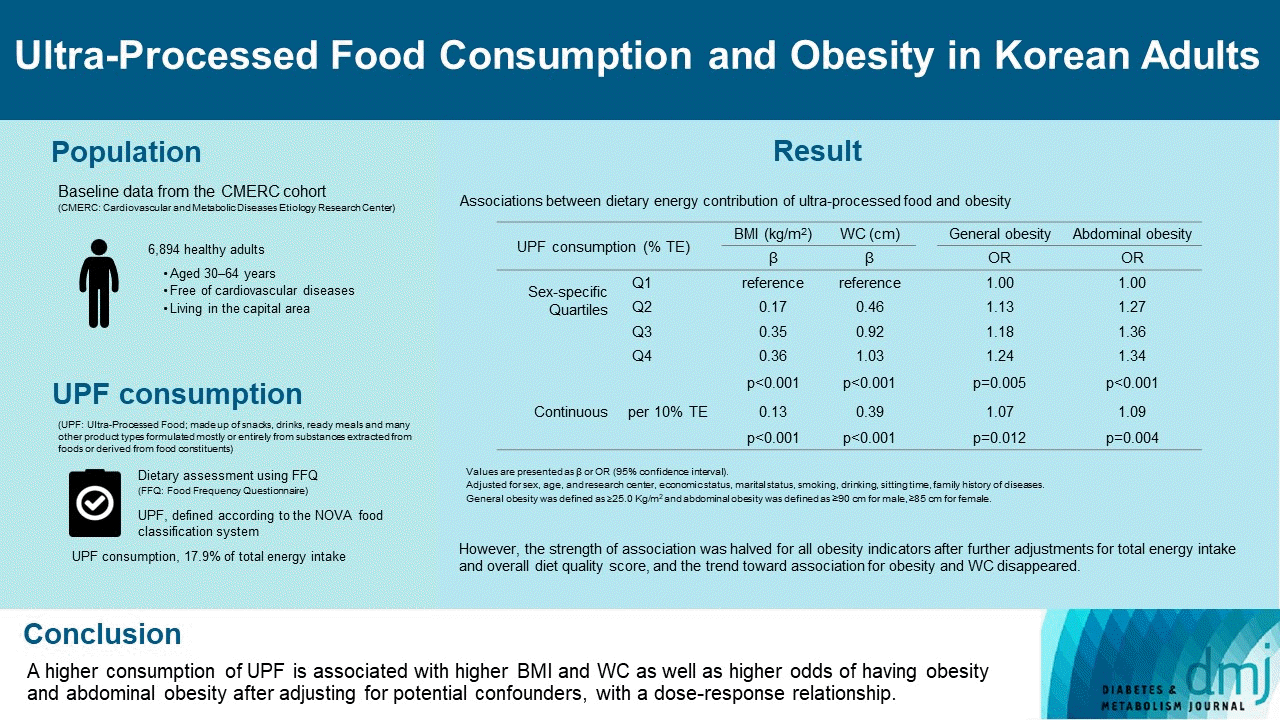
Results
Notes
CONFLICTS OF INTEREST
Dae Jung Kim has been associate editor of the Diabetes & Metabolism Journal since 2022. Hyeon Chang Kim has been statistical advisor of the Diabetes & Metabolism Journal since 2021. They were not involved in the review process of this article. Otherwise, there was no conflict of interest.
AUTHOR CONTRIBUTIONS
Conception or design: J.S.S., H.C.K.
Acquisition, analysis, or interpretation of data: J.S.S., K.H.H., D.J.K., H.C.K.
Drafting the work or revising: J.S.S., H.C.K.
Final approval of the manuscript: J.S.S., K.H.H., D.J.K., H.C.K.
FUNDING
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1I1A1-A01064904) and the Basic Research Program via the National Research Foundation of Korea funded by MSIT (NRF-2019R1-A4A1028155). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
Table 1.
| Characteristic | Total |
Sex-specific quartiles of UPF consumption, % Tea |
P valueb | |||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||
| Number | 6,894 | 1,724 | 1,730 | 1,717 | 1,723 | |
| Female sex | 4,523 (65.6) | 1,130 (65.6) | 1,136 (65.7) | 1,128 (65.7) | 1,129 (65.5) | - |
| Age, yr | 51.7±5.6 | 54.7±6.8 | 53.0±8.0 | 51.0±8.6 | 47.9±9.3 | <0.001 |
| 30–49 | 2,317 (33.6) | 315 (18.3) | 473 (27.3) | 649 (37.8) | 880 (51.1) | <0.001 |
| 50–64 | 4,577 (66.4) | 1,409 (81.7) | 1,257 (72.6) | 1,068 (62.2) | 843 (48.9) | |
| Economic status | ||||||
| High | 2,361 (34.3) | 609 (35.3) | 660 (38.2) | 575 (33.5) | 517 (30.0) | <0.001 |
| Middle | 3,120 (45.3) | 762 (44.2) | 756 (43.7) | 816 (47.5) | 786 (45.6) | |
| Low | 1,413 (20.5) | 353 (20.5) | 314 (18.2) | 326 (19.0) | 420 (24.4) | |
| Marital status | ||||||
| Married | 6,019 (87.3) | 1,529 (88.7) | 1,551 (89.6) | 1,491 (86.8) | 1,448 (84.0) | <0.001 |
| Unmarried | 875 (12.7) | 195 (11.3) | 179 (10.4) | 226 (13.2) | 275 (16.0) | |
| Smoking | ||||||
| Non-smoker | 4,796 (69.6) | 1,289 (74.8) | 1,248 (72.1) | 1,194 (69.5) | 1,065 (61.8) | <0.001 |
| Ex-smoker | 1,239 (18.0) | 333 (19.3) | 342 (19.8) | 291 (17.0) | 273 (15.8) | |
| Current smoker | 859 (12.5) | 102 (5.9) | 140 (8.1) | 232 (13.5) | 385 (22.3) | |
| Drinking | ||||||
| Non-drinker | 1,669 (24.2) | 528 (30.6) | 446 (25.8) | 378 (22.0) | 317 (18.4) | <0.001 |
| Ex-drinker | 260 (3.8) | 88 (5.1) | 66 (3.8) | 50 (2.9) | 56 (3.2) | |
| Current drinker | 4,965 (72.0) | 1,108 (64.3) | 1,218 (70.4) | 1,289 (75.1) | 1,350 (78.4) | |
| Sitting time, hr/day | 6.1±3.2 | 5.9±3.0 | 5.9±3.0 | 6.2±3.3 | 6.4±3.4 | 0.001 |
| Family history of diseasesc | 4,128 (59.9) | 1,039 (60.3) | 1,033 (59.7) | 1,027 (59.8) | 1,029 (59.7) | 0.985 |
| BMI, kg/m2 | 24.1±3.0 | 24.0±2.8 | 24.1±3.0 | 24.2±.1 | 24.1±3.2 | 0.312 |
| Obesityd | 2,444 (35.4) | 583 (33.8) | 615 (35.6) | 618 (36.0) | 628 (36.4) | 0.393 |
| Waist circumference, cm | 82.3±9.1 | 82.4±8.5 | 82.4±9.1 | 82.5±9.0 | 82.1±9.6 | 0.584 |
| Abdominal obesitye | 2,085 (30.2) | 488 (28.3) | 544 (31.4) | 544 (31.7) | 509 (29.5) | 0.096 |
| UPF consumption, %TE | 17.9±10.2 | 6.7±3.2 | 13.7±3.3 | 20.0±4.0 | 31.1±7.7 | <0.001 |
| Total energy, kcal/day | 2,176±640 | 1,968±558 | 2,184±612 | 2,259±665 | 2,293±667 | <0.001 |
| Carbohydrate, % TE | 69.4±7.0 | 71.4±6.9 | 69.2±6.7 | 68.7±6.9 | 68.4±7.1 | <0.001 |
| Protein, % TE | 13.3±2.2 | 13.2±2.2 | 13.6±2.1 | 13.4±2.2 | 12.9±2.3 | <0.001 |
| Fat, % TE | 17.3±5.3 | 15.5±5.2 | 17.2±5.0 | 17.9±5.1 | 18.7±5.2 | <0.001 |
| SFA, % TE | 4.7±1.6 | 3.8±1.3 | 4.5±1.3 | 5.0±1.5 | 5.6±1.7 | <0.001 |
| Cholesterol, mgq | 269.3±156.7 | 226.7±156.4 | 274.4±155.6 | 289.2±159.7 | 287±146.7 | <0.001 |
| Dietary fiber, g/day | 25.1±9.7 | 26.1±10.1 | 26.9±10 | 25.5±9.4 | 21.9±8.4 | <0.001 |
| Calcium, mg/day | 539.2±214.9 | 458.8±195.8 | 547.1±208.8 | 575.4±216.2 | 575.6±216.9 | <0.001 |
| Potassium, mg/day | 3,352±1,189 | 3,243±1,238 | 3,491±1,234 | 3,458±1,185 | 3,214±1,066 | <0.001 |
| Sodium, mg/day | 3,531±1,584 | 3,236±1,544 | 3,653±1,588 | 3,742±1,662 | 3,495±1,491 | <0.001 |
| Vitamin A, µgRAE | 713.5±342.5 | 690.9±354.2 | 759.2±361.3 | 737.7±332.6 | 666.3±312.1 | <0.001 |
| Thiamin, mg/day | 2.0±0.6 | 2.0±0.6 | 2.1±0.6 | 2.1±0.6 | 1.9±0.6 | <0.001 |
| Riboflavin, mg/day | 1.4±0.6 | 1.2±0.5 | 1.4±0.6 | 1.5±0.6 | 1.5±0.6 | <0.001 |
| Vitamin C, mg/day | 153.3±88.2 | 166.5±97 | 164.7±88.7 | 152.9±83.8 | 129.0±76.8 | <0.001 |
| KHEI score (0–100) | 72.8±11.2 | 73.4±10.2 | 75.5±9.9 | 73.7±10.9 | 68.8±12.4 | <0.001 |
| Adequacy (0–55) | 38.0±9.6 | 37.4±9.1 | 39.9±9.2 | 39.0±9.7 | 35.8±10.1 | <0.001 |
| Moderation (0–30) | 24.8±4.6 | 26.8±3.3 | 25.5±3.9 | 24.4±4.6 | 22.6±5.4 | <0.001 |
| Balance (0–15) | 10.0±3.7 | 9.2±4.0 | 10.1±3.6 | 10.3±3.5 | 10.5±3.5 | <0.001 |
Values are presented as number (%) or mean±standard deviation.
UPF, ultra-processed food; TE, total energy intake; BMI, body mass index; SFA, saturated fatty acid; KHEI, Korean Healthy Eating Index.
a The sex-specific cutoffs of UPF consumption (% TE) were 13.9%, 21.2%, and 28.4% in male and 9.0%, 14.5%, and 21.2% in female,
Table 2.
| Variable |
Sex-specific quartiles of UPF consumption, % TEa |
Per UPF consumption, 10% TE |
|||||
|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P trendb | β or OR | P valuec | |
| BMI, kg/m2 | |||||||
| Model 1 | 0 | 0.15 (–0.05 to 0.35) | 0.34 (0.14 to 0.54) | 0.36 (0.16 to 0.57) | <0.001 | 0.14 (0.07 to 0.22) | <0.001 |
| Model 2 | 0 | 0.17 (–0.02 to 0.37) | 0.35 (0.15 to 0.55) | 0.36 (0.15 to 0.56) | <0.001 | 0.13 (0.05 to 0.21) | <0.001 |
| Model 3 | 0 | 0.11 (–0.09 to 0.31) | 0.27 (0.07 to 0.47) | 0.27 (0.07 to 0.48) | 0.004 | 0.10 (0.02 to 0.18) | 0.009 |
| Model 4 | 0 | 0.13 (–0.06 to 0.33) | 0.26 (0.06 to 0.46) | 0.18 (–0.03 to 0.39) | 0.048 | 0.05 (–0.02 to 0.13) | 0.180 |
| Obesityd | |||||||
| Model 1 | 1 | 1.12 (0.97 to 1.29) | 1.18 (1.02 to 1.36) | 1.26 (1.08 to 1.46) | 0.002 | 1.08 (1.03 to 1.14) | 0.004 |
| Model 2 | 1 | 1.13 (0.98 to 1.30) | 1.18 (1.02 to 1.36) | 1.24 (1.07 to 1.45) | 0.005 | 1.07 (1.02 to 1.14) | 0.012 |
| Model 3 | 1 | 1.08 (0.94 to 1.26) | 1.12 (0.97 to 1.30) | 1.18 (1.01 to 1.38) | 0.032 | 1.06 (1.00 to 1.12) | 0.058 |
| Model 4 | 1 | 1.10 (0.95 to 1.27) | 1.11 (0.96 to 1.29) | 1.11 (0.95 to 1.30) | 0.186 | 1.02 (0.97 to 1.08) | 0.434 |
| Waist circumference, cm | |||||||
| Model 1 | 0 | 0.43 (–0.11 to 0.96) | 0.96 (0.42 to 1.50) | 1.18 (0.62 to 1.73) | <0.001 | 0.47 (0.27 to 0.68) | <0.001 |
| Model 2 | 0 | 0.46 (–0.08 to 0.99) | 0.92 (0.38 to 1.46) | 1.03 (0.46 to 1.60) | <0.001 | 0.39 (0.18 to 0.60) | <0.001 |
| Model 3 | 0 | 0.28 (–0.26 to 0.81) | 0.69 (0.14 to 1.24) | 0.79 (0.22 to 1.36) | 0.002 | 0.31 (0.10 to 0.52) | 0.004 |
| Model 4 | 0 | 0.36 (–0.18 to 0.89) | 0.66 (0.11 to 1.20) | 0.48 (–0.09 to 1.06) | 0.056 | 0.15 (–0.07 to 0.36) | 0.179 |
| Abdominal obesitye | |||||||
| Model 1 | 1 | 1.25 (1.08 to 1.45) | 1.37 (1.18 to 1.59) | 1.38 (1.18 to 1.61) | <0.001 | 1.12 (1.06 to 1.19) | <0.001 |
| Model 2 | 1 | 1.27 (1.09 to 1.47) | 1.36 (1.17 to 1.59) | 1.34 (1.14 to 1.57) | <0.001 | 1.11 (1.04 to 1.17) | <0.001 |
| Model 3 | 1 | 1.23 (1.06 to 1.43) | 1.31 (1.12 to 1.53) | 1.28 (1.09 to 1.51) | 0.002 | 1.09 (1.03 to 1.16) | 0.004 |
| Model 4 | 1 | 1.25 (1.07 to 1.45) | 1.30 (1.11 to 1.51) | 1.18 (1.00 to 1.38) | 0.040 | 1.04 (0.98 to 1.11) | 0.165 |
Values are presented as β or OR (95% confidence interval). Model 1: adjusted for sex, age, and research center; Model 2: adjusted for Model 1 plus economic status, marital status, smoking, drinking, sitting time, family history of diseases; Model 3: adjusted for Model 2 plus total energy intake; Model 4: adjusted for Model 3 plus Korean Healthy Eating Index.
UPF, ultra-processed food; TE, total energy intake; OR, odds ratio; BMI, body mass index.
Table 3.
| Variable | Mean±SD or n (%) |
Sex-specific quartiles of UPF consumption, % TEa |
Per UPF consumption, 10% TE |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P trendb | β or OR | P valuec | |||
| Male sex | |||||||||
| BMI, kg/m2 | 25.1±2.8 | 0 | 0.39 (0.06 to 0.71) | 0.18 (–0.15 to 0.51) | 0.52 (0.18 to 0.87) | 0.014 | 0.15 (0.03 to 0.26) | 0.011 | |
| Obesityd | 1,174 (49.5) | 1 | 1.26 (1.00 to 1.58) | 1.04 (0.82 to 1.32) | 1.31 (1.02 to 1.67) | 0.120 | 1.06 (0.98 to 1.15) | 0.176 | |
| Waist circumference, cm | 87.7±7.8 | 0 | 0.92 (0.04 to 1.80) | 0.72 (–0.19 to 1.62) | 1.57 (0.63 to 2.51) | 0.003 | 0.55 (0.24 to 0.87) | <0.001 | |
| Abdominal obesitye | 900 (38.0) | 1 | 1.35 (1.06 to 1.72) | 1.29 (1.00 to 1.65) | 1.45 (1.12 to 1.88) | 0.010 | 1.13 (1.04 to 1.23) | 0.004 | |
| Female sex | |||||||||
| BMI, kg/m2 | 23.6±3.0 | 0 | 0.07 (–0.18 to 0.31) | 0.46 (0.21 to 0.71) | 0.36 (0.10 to 0.62) | <0.001 | 0.15 (0.05 to 0.25) | 0.004 | |
| Obesity | 1,270 (28.1) | 1 | 1.05 (0.87 to 1.27) | 1.30 (1.08 to 1.57) | 1.26 (1.03 to 1.54) | 0.004 | 1.11 (1.03 to 1.20) | 0.009 | |
| Waist circumference, cm | 79.5±8.4 | 0 | 0.24 (–0.42 to 0.90) | 1.10 (0.43 to 1.78) | 1.03 (0.32 to 1.73) | <0.001 | 0.37 (0.10 to 0.64) | 0.008 | |
| Abdominal obesity | 1,185 (26.2) | 1 | 1.22 (1.01 to 1.48) | 1.44 (1.19 to 1.75) | 1.34 (1.09 to 1.66) | 0.001 | 1.11 (1.02 to 1.20) | 0.013 | |
| 30–49 years | |||||||||
| BMI, kg/m2 | 23.9±3.3 | 0 | 0.19 (–0.26 to 0.63) | 0.47 (0.05 to 0.90) | 0.52 (0.10 to 0.94) | 0.007 | 0.16 (0.03 to 0.30) | 0.014 | |
| Obesity | 788 (34.0) | 1 | 1.11 (0.80 to 1.53) | 1.30 (0.96 to 1.76) | 1.39 (1.03 to 1.87) | 0.016 | 1.10 (1.00 to 1.21) | 0.038 | |
| Waist circumference, cm | 80.9±9.6 | 0 | 0.19 (–1.00 to 1.34) | 0.85 (–0.25 to 1.96) | 1.16 (0.08 to 2.24) | 0.011 | 0.44 (0.10 to 0.78) | 0.011 | |
| Abdominal obesity | 570 (24.6) | 1 | 1.09 (0.77 to 1.55) | 1.17 (0.84 to 1.63) | 1.30 (0.94 to 1.79) | 0.082 | 1.12 (1.01 to 1.23) | 0.027 | |
| 50–64 years | |||||||||
| BMI, kg/m2 | 24.2±2.9 | 0 | 0.19 (–0.03 to 0.40) | 0.31 (0.08 to 0.54) | 0.34 (0.09 to 0.59) | 0.002 | 0.13 (0.04 to 0.22) | 0.007 | |
| Obesity | 1,656 (36.2) | 1 | 1.14 (0.97 to 1.34) | 1.15 (0.97 to 1.36) | 1.21 (1.01 to 1.46) | 0.040 | 1.07 (1.00 to 1.15) | 0.064 | |
| Waist circumference, cm | 83.1±8.7 | 0 | 0.58 (–0.01 to 1.18) | 1.00 (0.36 to 1.62) | 1.00 (0.31 to 1.68) | <0.001 | 0.38 (0.12 to 0.64) | 0.004 | |
| Abdominal obesity | 1,515 (33.1) | 1 | 1.32 (1.11 to 1.56) | 1.46 (1.22 to 1.74) | 1.32 (1.08 to 1.60) | <0.001 | 1.10 (1.03 to 1.18) | 0.008 | |
| Low diet qualityf | |||||||||
| BMI, kg/m2 | 24.4±3.2 | 0 | 0.25 (–0.06 to 0.56) | 0.31 (0.00 to 0.61) | 0.46 (0.16 to 0.76) | 0.003 | 0.14 (0.04 to 0.24) | 0.006 | |
| Obesity | 1,355 (39.2) | 1 | 1.25 (1.01 to 1.55) | 1.28 (1.04 to 1.58) | 1.42 (1.16 to 1.75) | 0.001 | 1.10 (1.03 to 1.18) | 0.007 | |
| Waist circumference, cm | 84.5±9.4 | 0 | 0.47 (–0.36 to 1.30) | 0.64 (–0.17 to 1.46) | 1.24 (0.44 to 2.05) | 0.003 | 0.48 (0.20 to 0.75) | <0.001 | |
| Abdominal obesity | 1,161 (33.6) | 1 | 1.22 (0.98 to 1.52) | 1.23 (0.99 to 1.52) | 1.40 (1.14 to 1.74) | 0.003 | 1.13 (1.05 to 1.21) | 0.001 | |
| High diet quality | |||||||||
| BMI, kg/m2 | 23.8±2.9 | 0 | 0.13 (–0.12 to 0.38) | 0.40 (0.14 to 0.67) | 0.18 (–0.11 to 0.48) | 0.040 | 0.10 (–0.02 to 0.23) | 0.096 | |
| Obesity | 1,089 (31.6) | 1 | 1.03 (0.84 to 1.26) | 1.10 (0.89 to 1.36) | 1.05 (0.83 to 1.32) | 0.520 | 1.03 (0.94 to 1.14) | 0.053 | |
| Waist circumference, cm | 81.2±8.6 | 0 | 0.54 (–0.15 to 1.24) | 1.24 (0.51 to 1.96) | 0.57 (–0.24 to 1.38) | 0.029 | 0.18 (–0.16 to 0.52) | 0.303 | |
| Abdominal obesity | 924 (26.8) | 1 | 1.36 (1.10 to 1.68) | 1.56 (1.26 to 1.95) | 1.15 (0.89 to 1.48) | 0.051 | 1.04 (0.94 to 1.15) | 0.435 | |
Values are presented as β or OR (95% confidence interval). All analyses adjusted for sex (not in sex-stratified analyses), age, research center, economic status, marital status, smoking, drinking, sitting time, family history of diseases.
SD, standard deviation; UPF, ultra-processed food; TE, total energy intake; OR, odds ratio; BMI, body mass index.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download