Abstract
To overcome the shortage of donor grafts in kidney transplantation (KT), the use of marginal grafts has evolved. However, prolonged cold ischemic time (CIT) is especially critical when using marginal grafts. Recently, hypothermic machine perfusion (HMP) has been used to overcome the negative effects of prolonged CIT, and we report the first use of HMP in Korea. The donor was a 58-year-old man with severe hypoxia (PaO2 <60 mmHg, FiO2 100%) for 9 hours prior to procurement. The patient’s kidneys were the only organs accepted for transplantation, and both kidneys were assigned to Jeju National University Hospital. After procurement, the right kidney was preserved using HMP immediately, and the left kidney was directly transplanted into a patient with a CIT of 2 hours 31 minutes. The second operation was performed following the first, using the right kidney graft that had been preserved by HMP for 10 hours and 30 minutes. Although postoperative graft function gradually recovered in both patients, the serum creatinine level decreased faster in the HMP patient. Neither patient showed signs of delayed graft function, and both were discharged without significant complications. The short-term outcomes in this transplantation of mate kidney grafts demonstrated that graft function can be safely preserved using HMP, and that HMP is beneficial in overcoming the negative effects of prolonged CIT.
Kidney transplantation (KT) is the preferred treatment for end-stage renal disease (ESRD), as it offers improved survival rates and quality of life compared to dialysis. However, a shortage of suitable donor organs remains a major limitation. Therefore, the use of marginal donors, such as donors after cardiac death (DCDs) or extended-criteria donors (ECDs), is increasing [1,2], and preservation methods that can compensate for marginal-quality grafts are attracting attention. Hypothermic machine perfusion (HMP) has gradually been accepted for the preservation of grafts that previously may not have been considered suitable for transplantation. HMP has also shown better short-term outcomes in transplanted kidney function compared to traditional static cold storage (SCS), especially in procured kidney grafts with a prolonged cold ischemic time (CIT). A CIT >18 hours has been reported to increase the incidence of acute tubular necrosis, delayed graft function (DGF), and graft failure, especially in ECDs [3].
During machine perfusion, the donor kidney is perfused with a solution that simulates blood flow and preserves the organ’s optimal physiology. In addition, machine perfusion theoretically improves graft quality during organ transportation by removing toxic metabolic products and supplying critical nutrients and oxygen. Studies have shown that machine perfusion can improve the survival rates of transplanted kidneys. In a randomized controlled trial conducted in the Netherlands, HMP reduced the risk of DGF and improved 1-year graft survival compared to cold storage [4]. Another study found that the use of machine perfusion in DCD kidneys improved 1-year graft survival from 42% to 63% [5].
KT has not been actively performed in patients with ESRD on Jeju Island in Korea because the island is geographically isolated. Since it is necessary to use public air transport to transfer deceased donor grafts from the mainland, prolonged CIT is inevitable. Furthermore, the overall status of the donor grafts assigned to KT recipients in Jeju is inferior to those assigned to mainland recipients. To overcome these problems in deceased donor KT (DDKT), Jeju National University Hospital (JNUH) introduced a portable HMP machine. By sharing the first case of DDKT using HMP in Korea, we hope to promote active DDKT on Jeju Island as well as the possible nationwide application of HMP.
This study was approved by the Institutional Review Board of JNUH (IRB No. JEJUNUH 2023-01-003). Written informed consent was obtained from the patients for publication of this case report and the accompanying images.
A 58-year-old man was admitted to JNUH with cardiac arrest due to asphyxia. The heart rhythm resumed, but he was diagnosed with brain death. The pneumonia-induced asphyxia worsened while in the intensive care unit (ICU). Even with maximal ventilator support (FiO2 100%), the patient’s oxygen saturation was marginal (85%–90%) during the last 8–9 hours before procurement. Because of the donor’s prolonged hypoxic condition, only the kidneys were accepted as suitable organs for procurement (Korean Kidney Donor Risk Index [K-KDPI] 50%). Both kidneys were assigned to JNUH. The left kidney graft was immediately transplanted into a 64-year-old man (patient 1) who had underlying hypertension and diabetes and had been on hemodialysis for 3 years. Anti-thymocyte globulin (ATG) induction was administered to the recipient based on his human leukocyte antigen matching status (A1B2DR2 mismatch). The left kidney, which weighed 220 g with a single renal artery and vein, was connected to the recipient's right external iliac artery (EIA).
During the first operation, the donor’s right kidney was preserved in the HPM instead of traditional SCS to protect the kidney from ischemic damage. For this purpose, a portable hypothermic perfusion machine (LifePort Kidney Transporter, Organ Recovery Systems Inc.) was used for 10 hours and 30 minutes (Fig. 1).
The second recipient was a 57-year-old male (patient 2) with hypertension and a history of left partial nephrectomy due to renal cell carcinoma (tumor/node/metastasis [TNM] stage 1A) and had been on dialysis for 1 year. ATG was used as induction therapy. The right kidney graft weighed 254 g with a single renal artery and vein and was anastomosed to the recipient's right EIA end-to-side fashion (Fig. 2). The total CIT was 12 hours and 34 minutes. All surgical procedures in both cases were uneventful (Table 1).
Both patients showed good postoperative progress with sufficient daily urine output from postoperative day (POD) 1 (Fig. 3A). The serum creatinine levels gradually decreased in both recipients, with a slightly faster rate of decrease in patient 2 (Fig. 3B). Doppler ultrasound on PODs 1 and 14 showed a normal range of the resistive index (RI) with good waveform (on POD 14, RI=0.84 in patient 1, and RI=0.74 in patient 2) (Fig. 4). Patient 1 complained of scrotal pain and swelling on POD 5, but the edema and pain gradually improved with conservative management. Patient 2 had no specific complications. Both patients were discharged on PODs 15 (HMP, patient 2) and 22 (SCS, patient 1) without significant events.
Machine perfusion refers to the mechanical process of circulating perfusate through organs extracorporeally. It is often used during KT procedures to preserve the condition of the procured kidney graft during transportation, ensuring that it remains viable and functional before it is transplanted into the recipient. The perfusion machine works by pumping cold preservation solutions through the organ, providing it with oxygen and nutrients. Additionally, machine perfusion prevents accumulation of the toxic metabolic products that are closely related to apoptosis in the organ, helping to keep it in good condition [6].
Machine perfusion includes HMP, which cools organs to a low temperature to slow metabolic activity, and normothermic machine perfusion (NMP), which maintains normal body temperature. Like conventional SCS, HMP is utilized more often in clinical practice than NMP due to its relative safety during organ delivery. Technical advances have also resulted in HMP devices small enough to be carried by one person during organ transportation [7].
The randomized controlled study conducted in the Netherlands (2005–2006) showed a lower rate of DGF and a higher 1-year graft survival in HMP than in SCS [4]. In a follow-up study published in March 2022, the incidence of DGF was significantly lower in the cohort studied after the introduction of machine perfusion than in the historical cohort that had used conventional SCS. Although there was no significant difference in graft survival between the two cohorts over a 2-year period, the safety and feasibility of HMP were sufficiently demonstrated. Therefore, by 2021, HMP was a national standard for all types of DDKT in the Netherlands [5].
In Korea, the portable hypothermic perfusion machine was first approved as an emergency relief medical device in September 2022, limited to Jeju Island and introduced to JNUH in December 2022. Because of the unique geographic situation of Jeju Island, as mentioned in the paper on Jeju’s first living donor kidney transplant [8], a CIT >18 hours frequently occurred in procured grafts, and was a critical factor that negatively impacted transplantation outcomes. Another study conducted in the United Kingdom reported that HMP showed superior early graft function compared to SCS despite a longer CIT [9]. Based on these results, the solution to overcoming the regional disadvantages of Jeju Island was the introduction of a portable perfusion machine. Now, when the transplantation team at JNUH goes to the mainland for kidney procurement, they can carry a portable machine and start machine perfusion immediately upon kidney procurement. It is expected that the use of machine perfusion during CIT will improve the performance of DDKT on Jeju Island by minimizing ischemic damage and maintaining healthier grafts.
In this case report, the donor was a 58-year-old man (K-KDPI 50%) with no specific medical history other than panic disorder who was admitted to JNUH with the return of spontaneous circulation after cardiac arrest due to asphyxia. Despite appropriate treatment, the patient was hypoxic (PaO2 50–60 mmHg with FiO2 100%) for the last 9 hours before procurement. Therefore, only his kidneys were accepted as suitable organs for procurement. Moreover, since there was only one team capable of KT at JNUH at the time, the second transplant operation could only be performed following completion of the first operation. Therefore, the second operation inevitably had a long CIT of >10 hours that could have a critical effect on the kidney transplant results for patient 2. As a workaround, we decided to perform machine perfusion on the kidney assigned to patient 2. After 10 hours and 30 minutes of machine perfusion, the second kidney was safely transplanted and patient 2 progressed as well as patient 1.
According to several studies, HMP has a lower incidence of DGF when compared to SCS as well as better early graft function in DCD KT [10-12]. This result was reinforced by the previously mentioned Dutch study, which compared the incidence of DGF in subgroups [4] and showed a higher preference for machine perfusion in DCD than in donation after brain death. Therefore, machine perfusion benefits the grafts from DCD, which are presumed to have greater exposure to hypoxic damage.
Several studies have shown that machine perfusion is more beneficial in the recovery of marginal donor grafts than SCS, even in cases with a CIT <16–18 hours. Notably, the recovery effect of machine perfusion in DCDs with marginal graft function can be even greater. In our case, the K-KDPI of the donor was 50%, and the hypoxic condition persisted for approximately 10 hours before organ procurement, much like a DCD donor. Therefore, even with a CIT of 10 hours, we decided to apply HMP for organ recovery purposes, which resulted in an encouraging short-term outcome.
Our case report showed that the kidney from a hypoxic donor with a prolonged CIT (compared to the average in Korea) could be transplanted safely by using HMP. Interest in DCD and ECD transplantation has also been rising in Korea since the first DCD KT in 2020 [13,14]. We believe that expanded use of machine perfusion will be needed, not only on Jeju Island, but nationwide in the transplantation of kidneys vulnerable to ischemic damage. Future large-scale studies of DDKT using machine perfusion should be conducted in Korea. In addition, parallel studies of molecular biology and pathology in relation to machine perfusion are necessary to prove its effectiveness.
The first use of HMP at JNUH in Korea demonstrated that graft function can be safely preserved using HMP, and that HMP is beneficial in overcoming prolonged CIT damage based on the short-term transplantation outcomes of kidney grafts from the same donor.
REFERENCES
1. Heilman RL, Smith ML, Kurian SM, Huskey J, Batra RK, Chakkera HA, et al. 2015; Transplanting kidneys from deceased donors with severe acute kidney injury. Am J Transplant. 15:2143–51. DOI: 10.1111/ajt.13260. PMID: 25808278.

2. Pascual J, Zamora J, Pirsch JD. 2008; A systematic review of kidney transplantation from expanded criteria donors. Am J Kidney Dis. 52:553–86. DOI: 10.1053/j.ajkd.2008.06.005. PMID: 18725015.

3. Hernandez D, Estupinan S, Perez G, Rufino M, Gonzalez-Posada JM, Luis D, et al. 2008; Impact of cold ischemia time on renal allograft outcome using kidneys from young donors. Transpl Int. 21:955–62. DOI: 10.1111/j.1432-2277.2008.00708.x. PMID: 18564990.

4. Moers C, Smits JM, Maathuis MH, Treckmann J, van Gelder F, Napieralski BP, et al. 2009; Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 360:7–19. DOI: 10.1056/NEJMoa0802289. PMID: 19118301.

5. Brat A, de Vries KM, van Heurn EW, Huurman VA, de Jongh W, Leuvenink HG, et al. 2022; Hypothermic machine perfusion as a national standard preservation method for deceased donor kidneys. Transplantation. 106:1043–50. DOI: 10.1097/TP.0000000000003845. PMID: 34172648. PMCID: PMC9038234.

6. Faro ML, Akhtar MZ, Boffa C, Ploeg R. 2015; Should pulsatile preservation be the gold standard in kidney transplantation? Curr Transpl Rep. 2:105–12. DOI: 10.1007/s40472-015-0063-8.

7. Yuan X, Theruvath AJ, Ge X, Floerchinger B, Jurisch A, García-Cardena G, et al. 2010; Machine perfusion or cold storage in organ transplantation: indication, mechanisms, and future perspectives. Transpl Int. 23:561–70. DOI: 10.1111/j.1432-2277.2009.01047.x. PMID: 20074082.

8. Shin YH, Lee T, Chang WB. 2022; The significance of the first living donor kidney transplantation in Jeju: a case report. Korean J Transplant. 36:231–5. DOI: 10.4285/kjt.22.0033. PMID: 36275993. PMCID: PMC9574429.

9. Guy A, McGrogan D, Inston N, Ready A. 2015; Hypothermic machine perfusion permits extended cold ischemia times with improved early graft function. Exp Clin Transplant. 13:130–7.
10. Jochmans I, Moers C, Smits JM, Leuvenink HG, Treckmann J, Paul A, et al. 2010; Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann Surg. 252:756–64. DOI: 10.1097/SLA.0b013e3181ffc256. PMID: 21037431.
11. Watson CJ, Wells AC, Roberts RJ, Akoh JA, Friend PJ, Akyol M, et al. 2010; Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. Am J Transplant. 10:1991–9. DOI: 10.1111/j.1600-6143.2010.03165.x. PMID: 20883534.

12. van der Vliet JA, Kievit JK, Héné RJ, Hilbrands LB, Kootstra G. 2001; Preservation of non-heart-beating donor kidneys: a clinical prospective randomised case-control study of machine perfusion versus cold storage. Transplant Proc. 33:847. DOI: 10.1016/S0041-1345(00)02343-5. PMID: 11267094.

13. Jeong E, Baik S, Park H, Oh J, Lee Y, Lee JM. 2021; First organ donation after circulatory death following withdrawal of life-sustaining treatment in Korea: a case report. J Korean Med Sci. 36:e171. DOI: 10.3346/jkms.2021.36.e171. PMID: 34128599. PMCID: PMC8203855.

14. Kim JM, Kim SJ, Joh JW, Kwon CH, Song S, Shin M, et al. 2011; Kidney donation after cardiac death in Korea. Transplant Proc. 43:1434–7. DOI: 10.1016/j.transproceed.2011.01.185. PMID: 21693213.

Fig. 1
(A) The renal artery of the right kidney is connected to the seal ring after trimming in the bench after procurement. (B) This seal ring will be connected to a cannula for the circulation of perfusate in the hypothermic pump machine. The renal vein is open in the perfusate container for draining out.
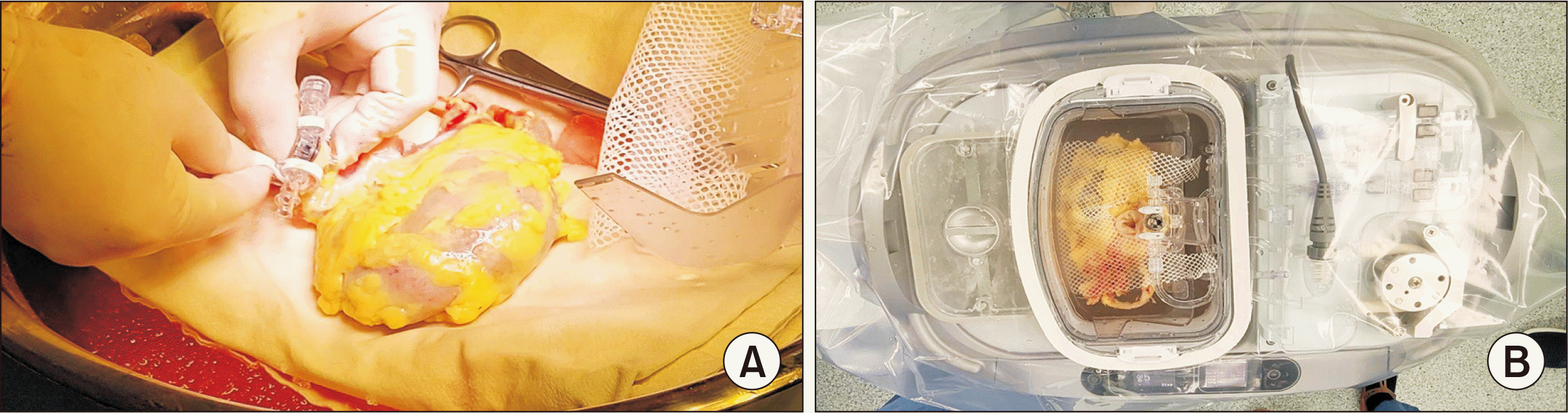
Fig. 2
The transplanted kidney right after anastomosis of the renal artery and vein. Some purple areas of the kidney quickly turned pink. This kidney was preserved in the hypothermic perfusion machine before transplantation.
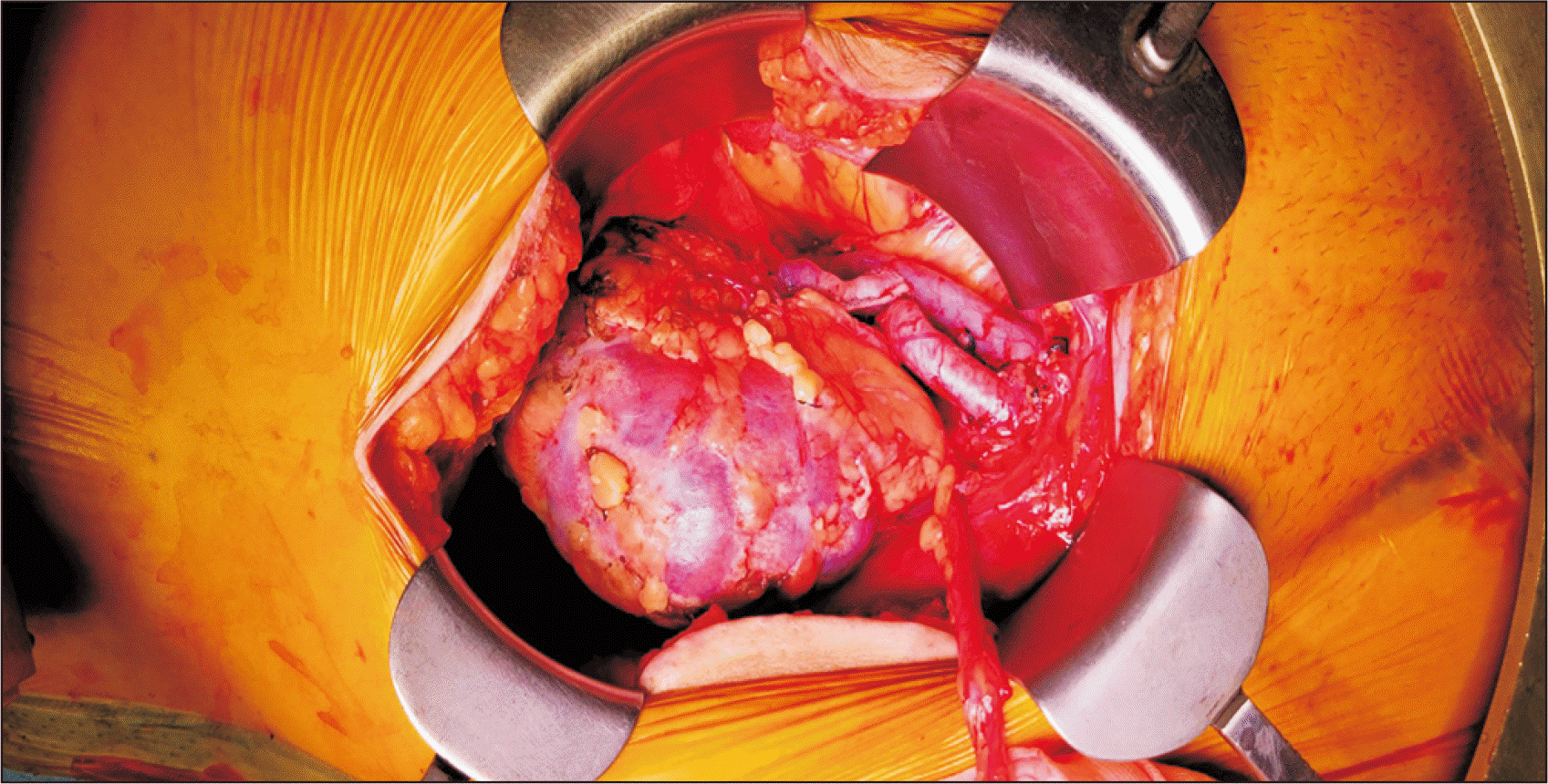
Fig. 3
The preoperative and postoperative (A) urine outputs and (B) creatinine levels of the two recipients. Both showed sufficient daily urine output from postoperative day (POD) 1. Although the serum creatinine level gradually decreased in both recipients, the rate of decrease was slightly faster in the recipient whose graft had been preserved via HMP before transplantation. SCS, static cold storage; HMP, hypothermic machine perfusion.
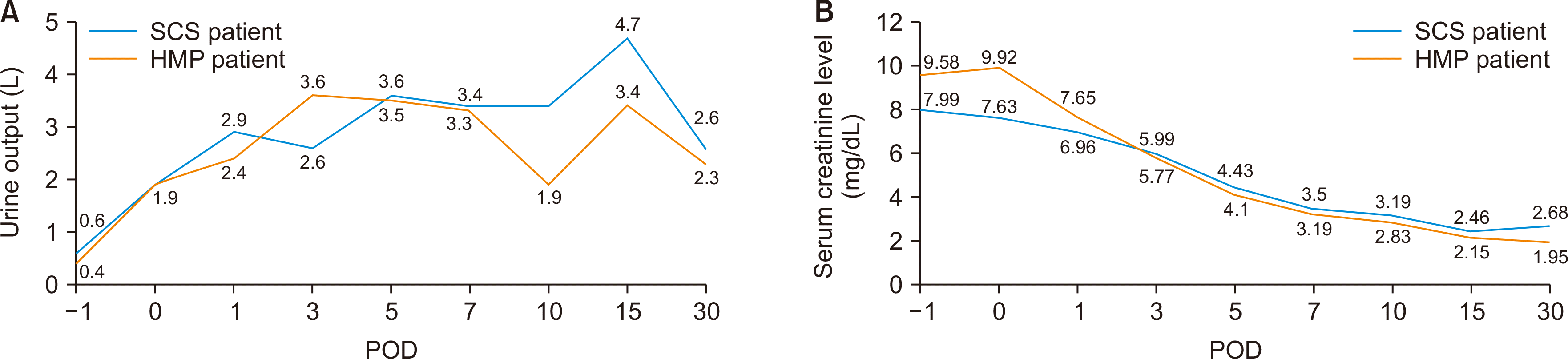
Fig. 4
(A) Kidney Doppler sonography on postoperative day 14 showing good vascular flow within the normal range of restrictive index (RI) in the static cold storage patient (RI, 0.84) and (B) the hypothermic machine perfusion patient (RI, 0.74).
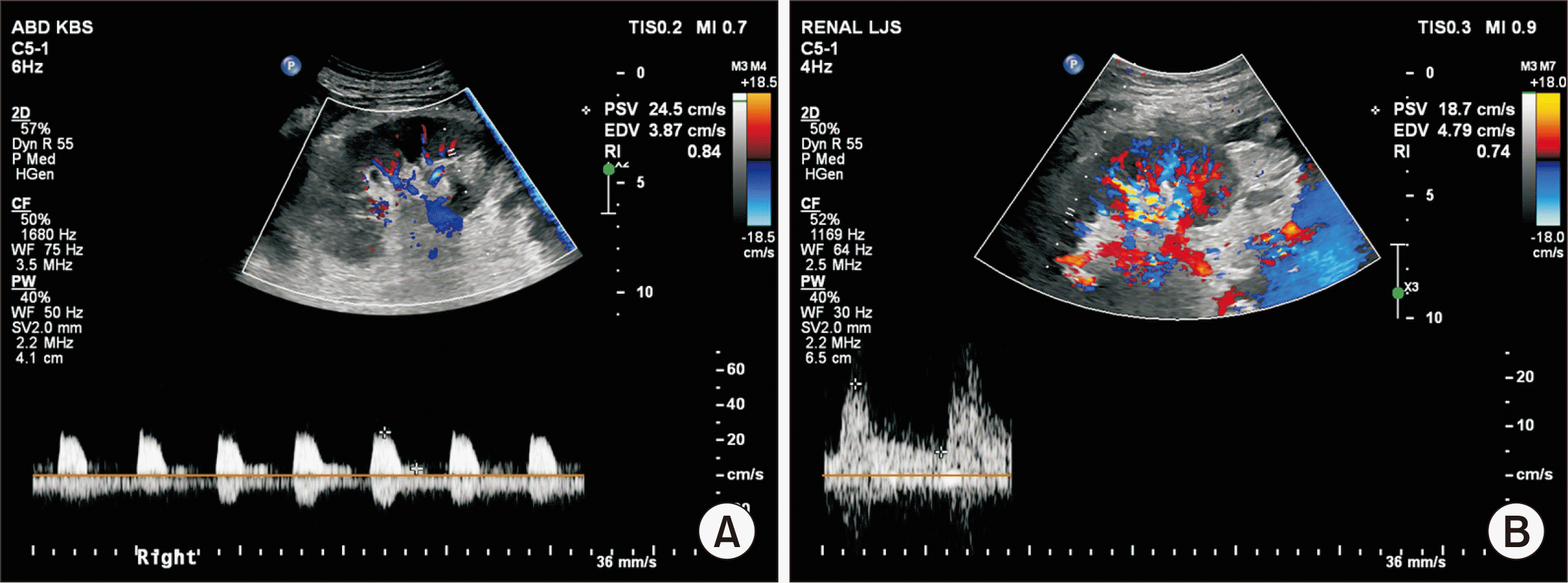
Table 1
Baseline characteristics of two kidney transplant recipients according to the donor graft preservation method




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download