Abstract
Clot waveform analysis (CWA) observes changes in transparency in a plasma sample based on clotting tests such as activated partial thromboplastin time (APTT), prothrombin time (PT), and thrombin time (TT). Evidence indicates that not only an abnormal waveform but also peak times and heights in derivative curves of CWA are useful for the evaluation of hemostatic abnormalities. Modified CWA, including the PT with APTT reagent, dilute PT (small amount of tissue factor [TF]-induced clotting factor IX [FIX] activation; sTF/FIXa), and dilute TT, has been proposed to evaluate physiological or pathological hemostasis. We review routine and modified CWA and their clinical applications. In CWA-sTF/FIXa, elevated peak heights indicate hypercoagulability in patients with cancer or thrombosis, whereas prolonged peak times indicate hypocoagulability in several conditions, including clotting factor deficiency and thrombocytopenia. CWA-dilute TT reflects the thrombin burst, whereas clot-fibrinolysis waveform analysis reflects both hemostasis and fibrinolysis. The relevance and usefulness of CWA-APTT and modified CWA should be further investigated in various diseases.
Clot waveform analysis (CWA) [1-5] is based on the activated partial thromboplastin time (APTT) [1-3], prothrombin time (PT) [4], or thrombin time (TT) [5] (CWA-APTT, CWA-PT, and CWA-TT, respectively) (Table 1). Although conventional clotting assays such as the APTT, PT, and TT are inexpensive, easy, and automated, enabling the measurement of multiple samples, they cannot visualize the clotting process. A thrombin generation test (TGT) [6] and thromboelastogram (TEG) [7] can help visualize the clotting process and provide more information than conventional clotting assays; however, these assays are expensive and are generally used only in research. Compared to the TGT and TEG [6, 7], the recently developed CWA using a fully automated optical coagulation analyzer can easily analyze hemostatic abnormalities.
CWA-APTT is most frequently used in the diagnosis of hemostatic abnormalities, such as hemophilia [8], inhibition of clotting factor [9], disseminated intravascular coagulation (DIC) [10], and lupus anticoagulant (LA) [7, 11]. There is still little evidence to support the clinical usefulness of CWA-PT and CWA-TT, as their short peak times make them more difficult to visualize and analyze. Therefore, CWA-dilute PT and CWA-dilute TT have been developed to evaluate physiological or pathological hemostasis [1, 6].
The first CWA using an MDA automated coagulation analyzer was reported in 2002, and a biphasic waveform was frequently observed in DIC or severely critically ill patients [10, 12]. The biphasic clot reaction curve reportedly was caused by a complex of C-reactive protein, very low-density lipoprotein, and calcium ions [13]. In addition, CWA-APTT can measure small amounts of clotting factor VIII (FVIII) activity [14], detect FVIII inhibitors [9], and evaluate the bleeding risk in patients undergoing major hepatobiliary and pancreatic surgery [15].
After visual evaluation of the fibrin formation curve (FFC) of blood coagulation on computer imaging by CWA, specific software programs can show the first derivative curve (1st DC) from the derivative of the FFC termed “velocity curve” and the 2nd DC from the derivative of the 1st DC termed “acceleration curve” [1]. There are three main mechanisms in hemostasis: the cascade system [16], thrombin burst [17, 18], and enhanced clotting factor activity on phospholipids of the platelet membrane [19], which are reflected by the FFC, 1st DC, and 2nd DC, respectively (Fig. 1). The peaks of the 1st and 2nd DCs of CWA-APTT continue from 28 seconds to 60 seconds (peak width); the peak width of the 1st and 2nd DCs reflects the thrombin burst, which has been investigated using the TGT [6] (Fig. 1).
A fully automated optical coagulation analyzer generally automatically enlarges the waveform to clear abnormal waveforms in CWA-APTT. For samples from patients with clotting factor deficiency or clotting factor inhibition [20, 21], DIC [10], liver dysfunction, or anticoagulant therapy [1], several abnormal waveforms (Fig. 2) still appear, suggesting that further examination for hemostatic abnormalities is required (Table 2). While the biphasic waveform, an abnormal waveform, first drew attention [10-12], a few specific abnormal waveforms have been identified for diseases associated with hemostatic abnormalities [10-12]. In addition, automatic waveform enlargement may cause peak time prolongation or peak height reduction to be missed, as many technicians and physicians may consider an abnormal peak time or height to be normal based on the automatic enlargement. Therefore, the analysis of peak times and heights is considered more useful for the evaluation of hemostatic abnormalities (Fig. 2 and Table 2).
Peak time, height, and width are associated with hemostatic abnormalities [15, 22]. A prolongation of the peak time, which reflects the cascade system, is useful for the detection of clotting factor deficiency or inhibition [20, 21], LA [11], or liver dysfunction, or the monitoring of patients undergoing anticoagulant therapy [23]. A decreased peak height indicates clotting factor deficiency or inhibition [20, 21] and reflects an increased risk of major bleeding [15]. A shortening of the peak time or an increased peak height may suggest hypercoagulability (Fig. 3). The detection of hypercoagulability in critically ill patients with coronavirus disease using CWA-APTT has been reported [24] (Fig. 3).
With CWA, the three abovementioned hemostatic mechanisms can be easily visualized. After the development of CWA-APTT, the mechanism of thrombin burst was recognized. That is, a small amount of thrombin activates not only fibrinogen but also FXI, FVIII, and FV. Activated FXI, FVIII, and FV activate downstream coagulation factors. The abovementioned activation cycle from thrombin to FXI continues for 10–30 seconds in the normal hemostatic system [22] (Fig. 3). However, in platelet-rich plasma (PRP), the thrombin burst is enhanced [25, 26]. CWA-APTT is performed using platelet-poor plasma (PPP). The 2nd DC reflects enhanced activity of clotting factors, particularly FVIII, on phospholipids, and is useful for the evaluation of hemophilia and diagnosis of LA [1].
Regarding the use of the CWA-APTT for monitoring anticoagulation therapy, an anti-Xa agent has been used as a prophylaxis against venous thromboembolism in orthopedic patients [23]. In addition, CWA-APTT has been used in enzyme kinetic analyses for anti-Xa agents, heparin, hirudin, and other drugs [27, 28].
As artificial phospholipids massively exist in APTT and PT reagents and most APTT reagents activate the contact pathway of coagulation, routine APTT or PT cannot evaluate a physiological coagulation reaction [22]. Furthermore, the various commercially available APTT reagents give different clotting times, suggesting the need for standardization of APTT reagents in the development of CWA [29]. In addition, platelets play important roles in hemostasis, the concentration of clotting factors on phospholipids of the platelet cell membrane [30], and the thrombin burst [17-19]. Routine APTT or PT using PPP cannot evaluate the effect of platelets on the coagulation system. When APTT is measured using PRP, the large amount of artificial phospholipids in the APTT reagent cancels out the effect of platelets on the coagulation reaction [22, 31].
The APTT test is carried out in two steps. In the first step, the large amount of artificial phospholipids activate FXII and finally generate activated FXI, FX, and FIX (FXIa, FXa, and FIXa, respectively). In the second step, upon the addition of Ca2+ solution, all coagulation reactions start simultaneously. These reactions are not physiological. For example, APTT is markedly shortened in hemophilic patients treated with emicizumab [32], and hemostatic ability and FVIII activity cannot be evaluated in these patients without an anti-neutral antibody for emicizumab [33]. In addition, CWA-APTT results are affected by the sampling condition, whereas the PT test shows a short clotting time and is difficult to use for thrombin burst evaluation. Therefore, dilute PT is used to evaluate the efficacy of oral anticoagulants [34].
Modified CWA is based on CWA-dilute PT [32, 35], dilute TT [5], and clot-fibrinolysis waveform analysis (CFWA) [36, 37]. CWA-dilute PT shows both the extrinsic and intrinsic pathways. CWA-dilute TT reflects not only fibrinogen activity but also the thrombin burst, and CFWA shows fibrinolytic activity as well as clotting activity.
CWA-dilute PT requires phospholipids to enlarge the waveform to facilitate a detailed analysis. CWA-PT with APTT [32] uses APTT reagent as a source of phospholipids, whereas the small amount of tissue factor (TF)-induced FIX activation (sTF/FIXa) assay uses PRP as a source of phospholipids [1, 35]. The use of dilute PT reagent as a source of TF prolongs the waveform. In particular, sTF/FIXa uses the concentration of dilute recombinant TF, which activates FIX but not FX [1, 35]; this assay shows physiological coagulation [22]. A small amount of TF activates FIX and finally generates a small amount of thrombin. The small amount of thrombin generated is not sufficient to induce fibrin formation, but it activates FXI, FVIII, and FV and initiates the activation cycle of clotting factors from FXIa to thrombin, leading to a thrombin burst.
CWA-PT with APTT is useful for evaluating small amounts of FVIII and FVIII inhibitors [3, 32, 37], whereas sTF/FIXa can measure FVIII activity [29] and evaluate hypercoagulability in patients with neoplasms [38]. As sTF/FIXa uses PRP, it has been used to evaluate hemostatic abnormalities in idiopathic thrombocytopenic purpura [31]. Finally, CWA-sTF/FIXa diagnoses hypercoagulability, which shortens the peak time and increases the peak height in patients with cancer or acute cerebral infarction, and hypocoagulability, which prolongs the peak time or decreases the peak height in patients with thrombocytopenia or clotting factor deficiency [31, 38] (Fig. 4).
Further investigations are needed to determine the optimal cutoff value of the peak height in CWA-sTF/FIXa for initiating anticoagulant therapy in patients with hypercoagulability. In PRP from patients with thrombocytopenia, decreased platelet membrane phospholipid concentrations may prolong the peak time and reduce the peak height in CWA-sTF/FIXa, suggesting that antiplatelet agents may not cause CWA abnormalities [1, 31].
CWA-dilute TT is conducted using 0.5 unit/mL thrombin [5]. A high concentration of thrombin activates fibrinogen to fibrin, suggesting that this assay is useful for the diagnosis of fibrinogen abnormalities [39], whereas a low concentration of thrombin activates FXI, FVIII, and FV, causing fibrin formation via the thrombin burst [5, 19]. Although evidence is limited, CWA-dilute TT may be able to evaluate upstream abnormalities in the clotting system as well as fibrinogen abnormalities. In addition, CWA-dilute TT visualizes the enhancement of the thrombin burst by platelets; the second peak of the velocity curve in CWA-TT is considered to show the enhancement of the thrombin burst by platelets [38] (Fig. 5). In patients with cancer, CWA-dilute TT reflects the enhancement of the thrombin burst by platelets [38], which may be the main cause of hypercoagulability in these patients. Therefore, over-enhancement of the thrombin burst may be a risk factor for thrombosis, which can be detected by CWA-dilute TT (Fig. 5).
The measurement of FVIII activity by APTT is difficult in patients with hemophilia A treated with emicizumab [40]; however, CWA-dilute TT can measure FVIII activity in plasma independent of the presence of emicizumab [41] (Fig. 6). Therefore, CWA-dilute TT may be useful for monitoring hemostasis in patients with hemophilia treated with emicizumab (Fig. 6).
CFWA combines CWA-APTT and a low concentration of tissue-type plasminogen activator (t-PA) to examine both coagulation and the fibrinolysis system [36, 37]. This assay can reveal a hyperfibrinolytic state in patients with DIC [42]. It shows two peaks: a positive fibrin formation peak caused by APTT and a negative peak caused by t-PA-induced fibrinolysis. Although a shortened or increased second peak reflects increased hyperfibrinolysis, the second peak may be affected by the first peak, which shows coagulability. Because of the addition of t-PA, this assay cannot show intrinsic fibrinolysis.
Hypercoagulability based on CWA-APTT and sTF/FIXa has been reported in patients with acute cerebral infarction [43] and malignant neoplasms [38], which has led to the establishment of a CWA cutoff value for thrombosis. A CWA cutoff value for major bleeding is being established. Thrombosis or major bleeding can be prevented using CWA-APTT or sTF/FIXa. CWA-TT and sTF/FIXa allow easy monitoring of hemophilia A treated with emicizumab, which is difficult to monitor using conventional APTT.
CWA can show abnormal waveforms, peak times, and peak heights and increases the ability to diagnose various hemostatic abnormalities. Modified CWA improves the routine clotting time assay to allow the examination of complex hemostatic abnormalities, such as hypercoagulability, major bleeding risk, and fibrinolysis, as well as the monitoring of various anticoagulant agents.
ACKNOWLEDGEMENTS
We thank Nisii H and Sakano Y and Instrumentation Laboratory (Bedford, MA, USA) for their kind support in performing CWA.
Notes
REFERENCES
1. Wada H, Matsumoto T, Ohishi K, Shiraki K, Shimaoka M. 2020; Update on the clot waveform analysis. Clin Appl Thromb Hemost. 26:1076029620912027. DOI: 10.1177/1076029620912027. PMID: 32862666. PMCID: PMC7466886.

2. Sevenet PO, Depasse F. 2017; Clot waveform analysis: where do we stand in 2017? Int J Lab Hematol. 39:561–8. DOI: 10.1111/ijlh.12724. PMID: 28876509.

3. Nogami K. Clot waveform analysis for monitoring hemostasis. Semin Thromb Hemost. 2022; doi: 10.1055/s-0042-1756706 (Online ahead of print). DOI: 10.1055/s-0042-1756706.

4. Arai S, Kamijo T, Hayashi F, Shinohara S, Arai N, Sugano M, et al. 2021; Screening method for congenital dysfibrinogenemia using clot waveform analysis with the Clauss method. Int J Lab Hematol. 43:281–9. DOI: 10.1111/ijlh.13358. PMID: 33030793.

5. Wada H, Ichikawa Y, Ezaki M, Matsumoto T, Yamashita Y, Shiraki K, et al. 2021; The reevaluation of thrombin time using a clot waveform analysis. J Clin Med. 10:4840. DOI: 10.3390/jcm10214840. PMID: 34768360. PMCID: PMC8585015.

6. Wu Y, Lu Y, Zhang J. 2023; Thrombin generation assay: the present and the future. Blood Coagul Fibrinolysis. 34:1–7. DOI: 10.1097/MBC.0000000000001170. PMID: 36598375.

7. Thakkar M, Rose A, Bednarz B. 2022; Thromboelastography in microsurgical reconstruction: a systematic review. JPRAS Open. 32:24–33. DOI: 10.1016/j.jpra.2021.12.005. PMID: 35242985. PMCID: PMC8857410.

8. Shima M. 2004; Understanding the hemostatic effects of recombinant factor VIIa by clot wave form analysis. Semin Hematol. 41(Suppl 1):125–31. DOI: 10.1053/j.seminhematol.2003.11.021. PMID: 14872433.

9. Matsumoto T, Nogami K, Shima M. 2017; A combined approach using global coagulation assays quickly differentiates coagulation disorders with prolonged aPTT and low levels of FVIII activity. Int J Hematol. 105:174–83. DOI: 10.1007/s12185-016-2108-x. PMID: 27730530.

10. Toh CH, Giles AR. 2002; Waveform analysis of clotting test optical profiles in the diagnosis and management of disseminated intravascular coagulation (DIC). Clin Lab Haematol. 24:321–7. DOI: 10.1046/j.1365-2257.2002.00457.x. PMID: 12452811.

11. Tokutake T, Baba H, Shimada Y, Takeda W, Sato K, Hiroshima Y, et al. 2016; Exogenous magnesium chloride reduces the activated partial thromboplastin times of lupus anticoagulant-positive patients. PLOS ONE. 11:e0157835. DOI: 10.1371/journal.pone.0157835. PMID: 27355205. PMCID: PMC4927146.

12. Matsumoto T, Wada H, Nishioka Y, Nishio M, Abe Y, Nishioka J, et al. 2006; Frequency of abnormal biphasic aPTT clot waveforms in patients with underlying disorders associated with disseminated intravascular coagulation. Clin Appl Thromb Hemost. 12:185–92. DOI: 10.1177/107602960601200206. PMID: 16708120.

13. Toh CH, Samis J, Downey C, Walker J, Becker L, Brufatto N, et al. 2002; Biphasic transmittance waveform in the APTT coagulation assay is due to the formation of a Ca(++)-dependent complex of C-reactive protein with very-low-density lipoprotein and is a novel marker of impending disseminated intravascular coagulation. Blood. 100:2522–9. DOI: 10.1182/blood.V100.7.2522. PMID: 12239165.

14. Shima M, Matsumoto T, Fukuda K, Kubota Y, Tanaka I, Nishiya K, et al. 2002; The utility of activated partial thromboplastin time (aPTT) clot waveform analysis in the investigation of hemophilia A patients with very low levels of factor VIII activity (FVIII:C). Thromb Haemost. 87:436–41. DOI: 10.1055/s-0037-1613023. PMID: 11916076.

15. Maeda K, Wada H, Shinkai T, Tanemura A, Matsumoto T, Mizuno S. 2021; Evaluation of hemostatic abnormalities in patients who underwent major hepatobiliary pancreatic surgery using activated partial thromboplastin time-clot waveform analysis. Thromb Res. 201:154–60. DOI: 10.1016/j.thromres.2021.03.030. PMID: 33862519.

16. Winter WE, Greene DN, Beal SG, Isom JA, Manning H, Wilkerson G, et al. 2020; Clotting factors: clinical biochemistry and their roles as plasma enzymes. Adv Clin Chem. 94:31–84. DOI: 10.1016/bs.acc.2019.07.008. PMID: 31952574.

17. Al-Amer OM. 2022; The role of thrombin in haemostasis. Blood Coagul Fibrinolysis. 33:145–8. DOI: 10.1097/MBC.0000000000001130. PMID: 35239615.
18. Monroe DM, Hoffman M, Roberts HR. 2002; Platelets and thrombin generation. Arterioscler Thromb Vasc Biol. 22:1381–9. DOI: 10.1161/01.ATV.0000031340.68494.34. PMID: 12231555.

19. Konstantinidi A, Sokou R, Parastatidou S, Lampropoulou K, Katsaras G, Boutsikou T, et al. 2019; Clinical application of thromboelastography/thromboelastometry (TEG/TEM) in the neonatal population: a narrative review. Semin Thromb Hemost. 45:449–57. DOI: 10.1055/s-0039-1692210. PMID: 31195422.

20. Matsumoto T, Wada H, Fujimoto N, Toyoda J, Abe Y MR, Ohishi K, et al. 2018; An evaluation of the activated partial thromboplastin time waveform. Clin Appl Thromb Hemost. 24:764–70. DOI: 10.1177/1076029617724230. PMID: 28884611. PMCID: PMC6714873.

21. Katayama H, Matsumoto T, Wada H, Fujimoto N, Toyoda J, Abe Y, et al. 2018; An evaluation of hemostatic abnormalities in patients with hemophilia according to the activated partial thromboplastin time waveform. Clin Appl Thromb Hemost. 24:1170–6. DOI: 10.1177/1076029618757344. PMID: 29439640. PMCID: PMC6714760.

22. Wada H, Shiraki K, Matsumoto T, Ohishi K, Shimpo H, Shimaoka M. 2020; Effects of platelet and phospholipids on clot formation activated by a small amount of tissue factor. Thromb Res. 193:146–53. DOI: 10.1016/j.thromres.2020.06.018. PMID: 32559572.

23. Hasegawa M, Tone S, Wada H, Naito Y, Matsumoto T, Yamashita Y, et al. 2021; The evaluation of hemostatic abnormalities using a CWA-small amount tissue factor induced FIX activation assay in major orthopedic surgery patients. Clin Appl Thromb Hemost. 27:10760296211012094. DOI: 10.1177/10760296211012094. PMID: 34027710. PMCID: PMC8150457.

24. Fan BE, Ng J, Chan SSW, Christopher D, Tso ACY, Ling LM, et al. 2021; COVID-19 associated coagulopathy in critically ill patients: a hypercoagulable state demonstrated by parameters of haemostasis and clot waveform analysis. J Thromb Thrombolysis. 51:663–74. DOI: 10.1007/s11239-020-02318-x. PMID: 33098540. PMCID: PMC7584863.

25. Tripodi A. 2016; Thrombin generation assay and its application in the clinical laboratory. Clin Chem. 62:699–707. DOI: 10.1373/clinchem.2015.248625. PMID: 26955824.

26. Bendetowicz AV, Kai H, Knebel R, Caplain H, Hemker HC, Lindhout T, et al. 1994; The effect of subcutaneous injection of unfractionated and low molecular weight heparin on thrombin generation in platelet rich plasma-a study in human volunteers. Thromb Haemost. 72:705–12. DOI: 10.1055/s-0038-1648946. PMID: 7900078.

27. Wakui M, Fujimori Y, Nakamura S, Kondo Y, Kuroda Y, Oka S, et al. 2019; Distinct features of bivalent direct thrombin inhibitors, hirudin and bivalirudin, revealed by clot waveform analysis and enzyme kinetics in coagulation assays. J Clin Pathol. 72:817–24. DOI: 10.1136/jclinpath-2019-205922. PMID: 31366633.

28. Wakui M, Fujimori Y, Katagiri H, Nakamura S, Kondo Y, Kuroda Y, et al. 2019; Assessment of in vitro effects of direct thrombin inhibitors and activated factor X inhibitors through clot waveform analysis. J Clin Pathol. 72:244–50. DOI: 10.1136/jclinpath-2018-205517. PMID: 30518630.

29. Wada H, Shiraki K, Matsumoto T, Ohishi K, Shimpo H, Sakano Y, et al. 2021; The evaluation of APTT reagents in reference plasma, recombinant FVIII products; Kovaltry® and Jivi® using CWA, including sTF/7FIX assay. Clin Appl Thromb Hemost. 27:1076029620976913. DOI: 10.1177/1076029620976913. PMID: 33606948. PMCID: PMC7900842.
30. Bourguignon A, Tasneem S, Hayward CPM. 2022; Update on platelet procoagulant mechanisms in health and in bleeding disorders. Int J Lab Hematol. 44(Suppl 1):89–100. DOI: 10.1111/ijlh.13866. PMID: 36074709.

31. Wada H, Ichikawa Y, Ezaki M, Shiraki K, Moritani I, Yamashita Y, et al. 2021; Clot waveform analysis demonstrates low blood coagulation ability in patients with idiopathic thrombocytopenic purpura. J Clin Med. 10:5987. DOI: 10.3390/jcm10245987. PMID: 34945283. PMCID: PMC8705019.

32. Nogami K, Matsumoto T, Tabuchi Y, Soeda T, Arai N, Kitazawa T, et al. 2018; Modified clot waveform analysis to measure plasma coagulation potential in the presence of the anti-factor IXa/factor X bispecific antibody emicizumab. J Thromb Haemost. 16:1078–88. DOI: 10.1111/jth.14022. PMID: 29645406.
33. Ogiwara K, Furukawa S, Shinohara S, Tabuchi Y, Arai N, Noguchi-Sasaki M, et al. 2023; Anti-idiotype monoclonal antibodies against emicizumab enable accurate procoagulant and anticoagulant assays, irrespective of the test base, in the presence of emicizumab. Haemophilia. 29:329–35. DOI: 10.1111/hae.14662. PMID: 36137299.

34. Kumano O, Suzuki S, Yamazaki M, An Y, Yasaka M, Ieko M, et al. 2023; Evaluation of newly-developed modified diluted prothrombin time reagent in non-valvular atrial fibrillation patients with direct oral anticoagulants: a comparative study with conventional reagents. Int J Lab Hematol. 45:119–25. DOI: 10.1111/ijlh.13971. PMID: 36114152.

35. Wada H, Matsumoto T, Yamashita Y, Ohishi K, Ikejiri M, Katayama N. 2019; Routine measurements of factor VIII activity and inhibitor titer in the presence of emicizumab utilizing anti-idiotype monoclonal antibodies: comment. J Thromb Haemost. 17:555–6. DOI: 10.1111/jth.14395. PMID: 30672649.

36. Onishi T, Shimonishi N, Takeyama M, Furukawa S, Ogiwara K, Nakajima Y, et al. 2022; The balance of comprehensive coagulation and fibrinolytic potential is disrupted in patients with moderate to severe COVID-19. Int J Hematol. 115:826–37. DOI: 10.1007/s12185-022-03308-w. PMID: 35171446. PMCID: PMC8852977.

37. Nogami K, Matsumoto T, Sasai K, Ogiwara K, Arai N, Shima M. 2019; A novel simultaneous clot-fibrinolysis waveform analysis for assessing fibrin formation and clot lysis in haemorrhagic disorders. Br J Haematol. 187:518–29. DOI: 10.1111/bjh.16111. PMID: 31335970.

38. Kobayashi M, Wada H, Fukui S, Mizutani H, Ichikawa Y, Shiraki K, et al. 2021; A clot waveform analysis showing a hypercoagulable state in patients with malignant neoplasms. J Clin Med. 10:5352. DOI: 10.3390/jcm10225352. PMID: 34830633. PMCID: PMC8618625.

39. Menegatti M, Palla R. 2020; Clinical and laboratory diagnosis of rare coagulation disorders (RCDs). Thromb Res. 196:603–8. DOI: 10.1016/j.thromres.2019.09.006. PMID: 31515069.

40. Lowe A, Kitchen S, Jennings I, Kitchen DP, Woods TAL, Walker ID. 2020; Effects of emicizumab on APTT, FVIII assays and FVIII inhibitor assays using different reagents: results of a UK NEQAS proficiency testing exercise. Haemophilia. 26:1087–91. DOI: 10.1111/hae.14177. PMID: 33094895.

41. Wada H, Shiraki K, Matsumoto T, Suzuki K, Yamashita Y, Tawara I, et al. 2022; A clot waveform analysis of thrombin time using a small amount of thrombin is useful for evaluating the clotting activity of plasma independent of the presence of emicizumab. J Clin Med. 11:6142. DOI: 10.3390/jcm11206142. PMID: 36294464. PMCID: PMC9605059.

42. Onishi T, Ishihara T, Nogami K. 2021; Coagulation and fibrinolysis balance in disseminated intravascular coagulation. Pediatr Int. 63:1311–8. DOI: 10.1111/ped.14684. PMID: 33660897.

43. Kamon T, Horie S, Inaba T, Ito N, Shiraki K, Ichikawa Y, et al. 2023; The detection of hypercoagulability in patients with acute cerebral infarction using a clot waveform analysis. Clin Appl Thromb Hemost. 29:10760296231161591. DOI: 10.1177/10760296231161591. PMID: 36872898. PMCID: PMC9989368.
Fig. 1
Normal CWA-APTT with a FFC, 1st DC, and 2nd DC and three mechanisms of hemostasis. The horizontal double-headed arrow indicates peak width (period of thrombin burst).
Abbreviations: FFC, fibrin formation curve; 1st DC, first derivative curve; 2nd DC, second derivative curve; TF, tissue factor; PLs, phospholipids; FXI, clotting factor XI; FXIa, activated FXI.
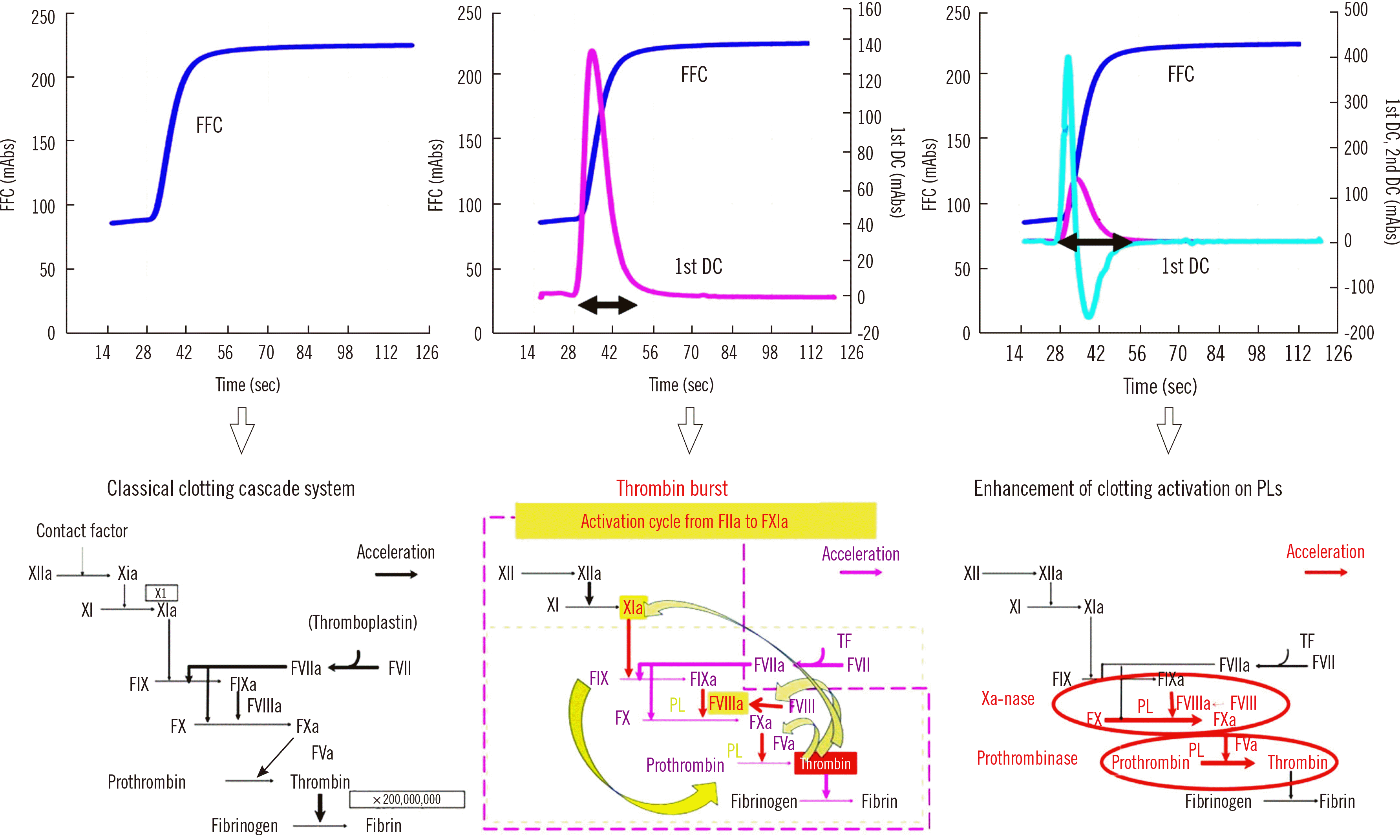
Fig. 2
Abnormal waveform in CWA-APTT. The fibrin formation curve is indicated in blue, the 1st DC in red, and the 2nd DC in green. The 1st and 2nd DCs show a bpw.
Abbreviations: CWA, clot waveform analysis; APTT, activated partial thromboplastin time; bpw, biphasic waveform; DC, derivative curve.
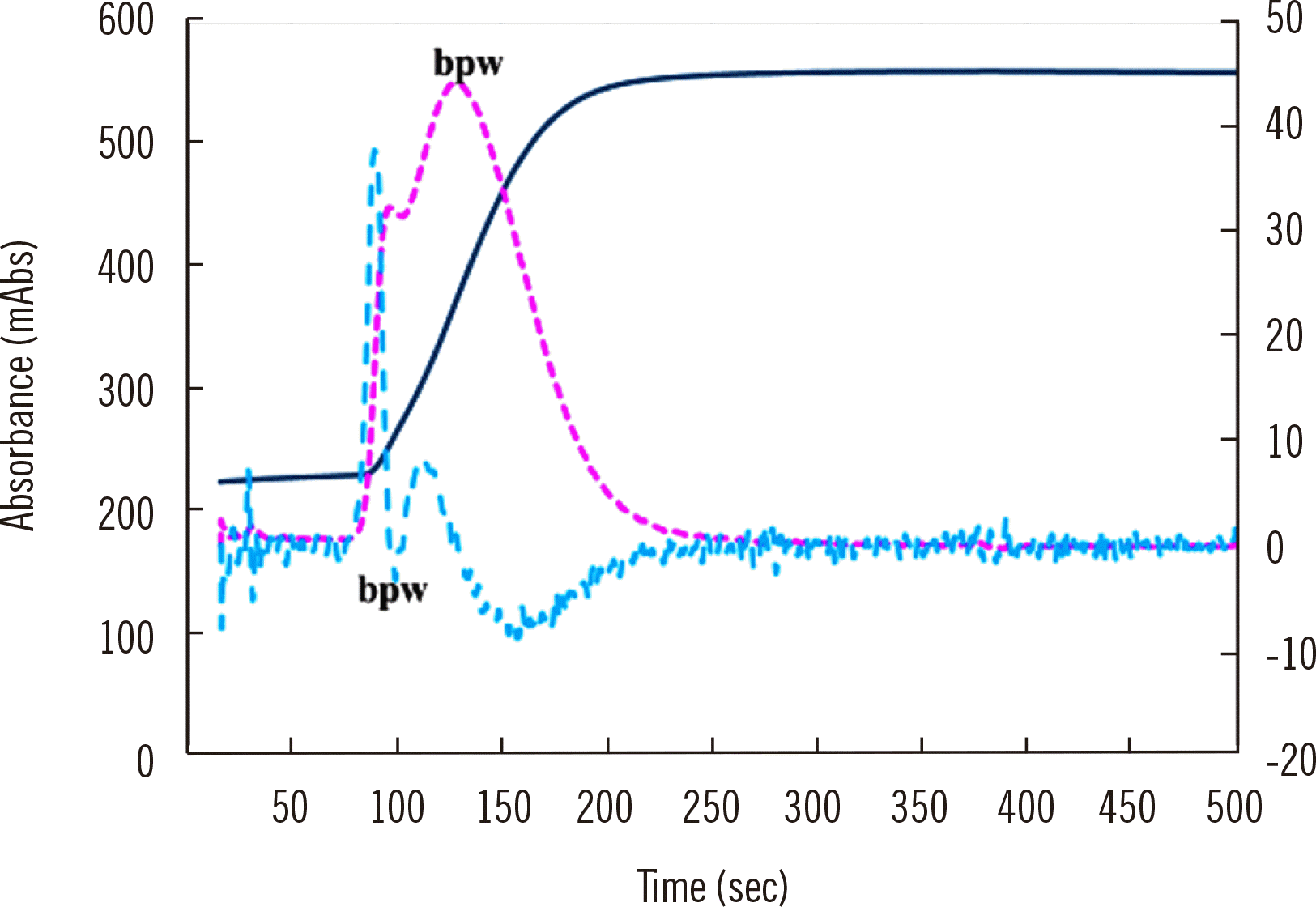
Fig. 3
Abnormal APTT and peak height in CWA-APTT. The solid line represents a patient sample, and the dotted line represents a sample from a healthy volunteer. A decreased peak height indicates a major bleeding risk, whereas an increased peak height indicates hypercoagulability.
Abbreviations: APTT, activated partial thromboplastin time; CWA, clot waveform analysis.
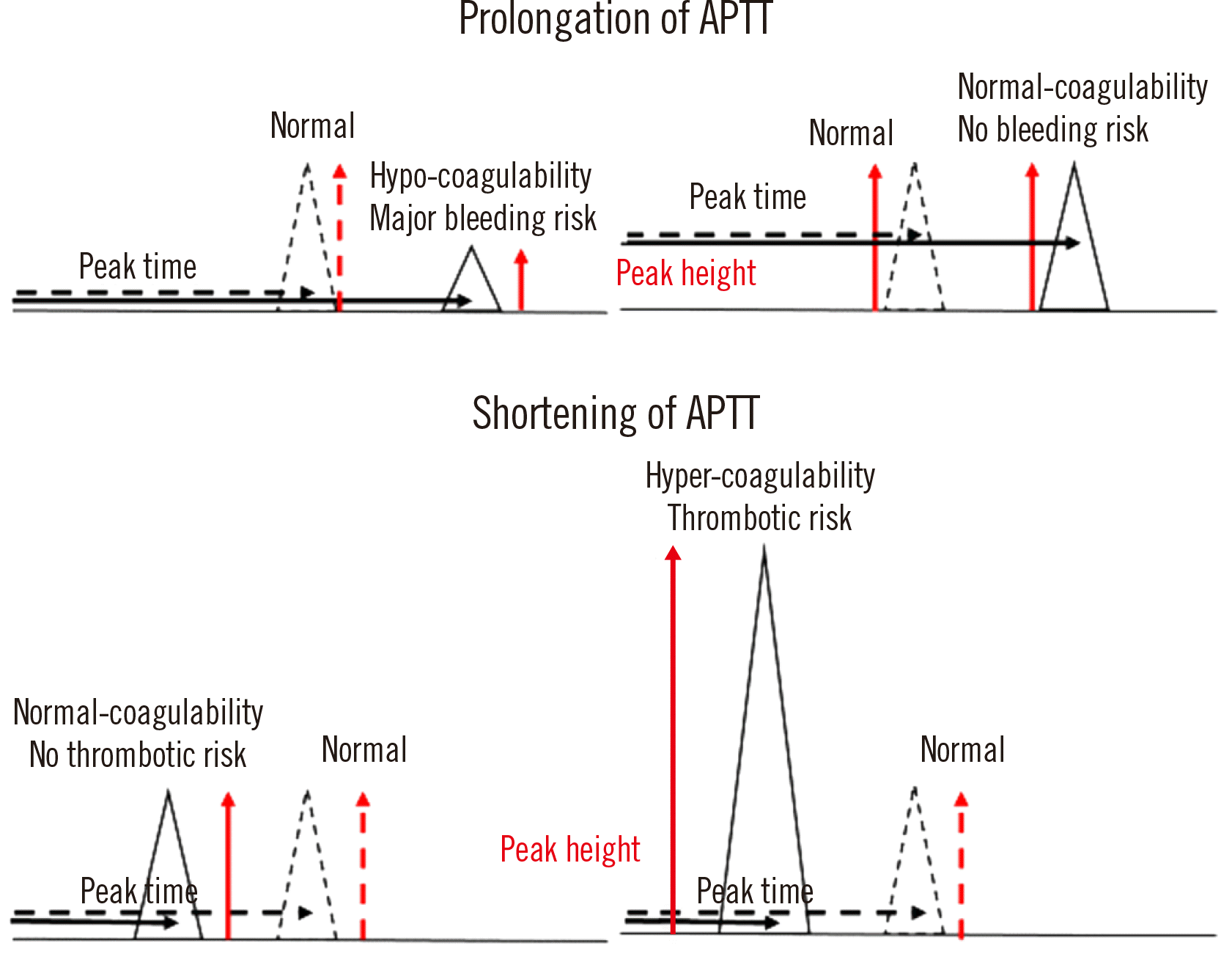
Fig. 4
CWA-sTF/FIXa of a patient with a neoplasm and acute cerebral infarction (A) and a patient with thrombocytopenia due to aplastic anemia (B). The fibrin formation curve is indicated in dark blue, the 1st DC (velocity) in pink, and the 2nd DC (acceleration) in light blue. The solid line represents a patient sample, and the dotted line represents a sample from a healthy volunteer. The peak heights of the three curves were significantly higher for patient (A) than for the healthy volunteer and significantly lower for patient (B) than for the healthy volunteer.
Abbreviations: CWA, clot waveform analysis; DC, derivative curve; sTF/FIXa, small amount of tissue factor-induced clotting factor IX activation.
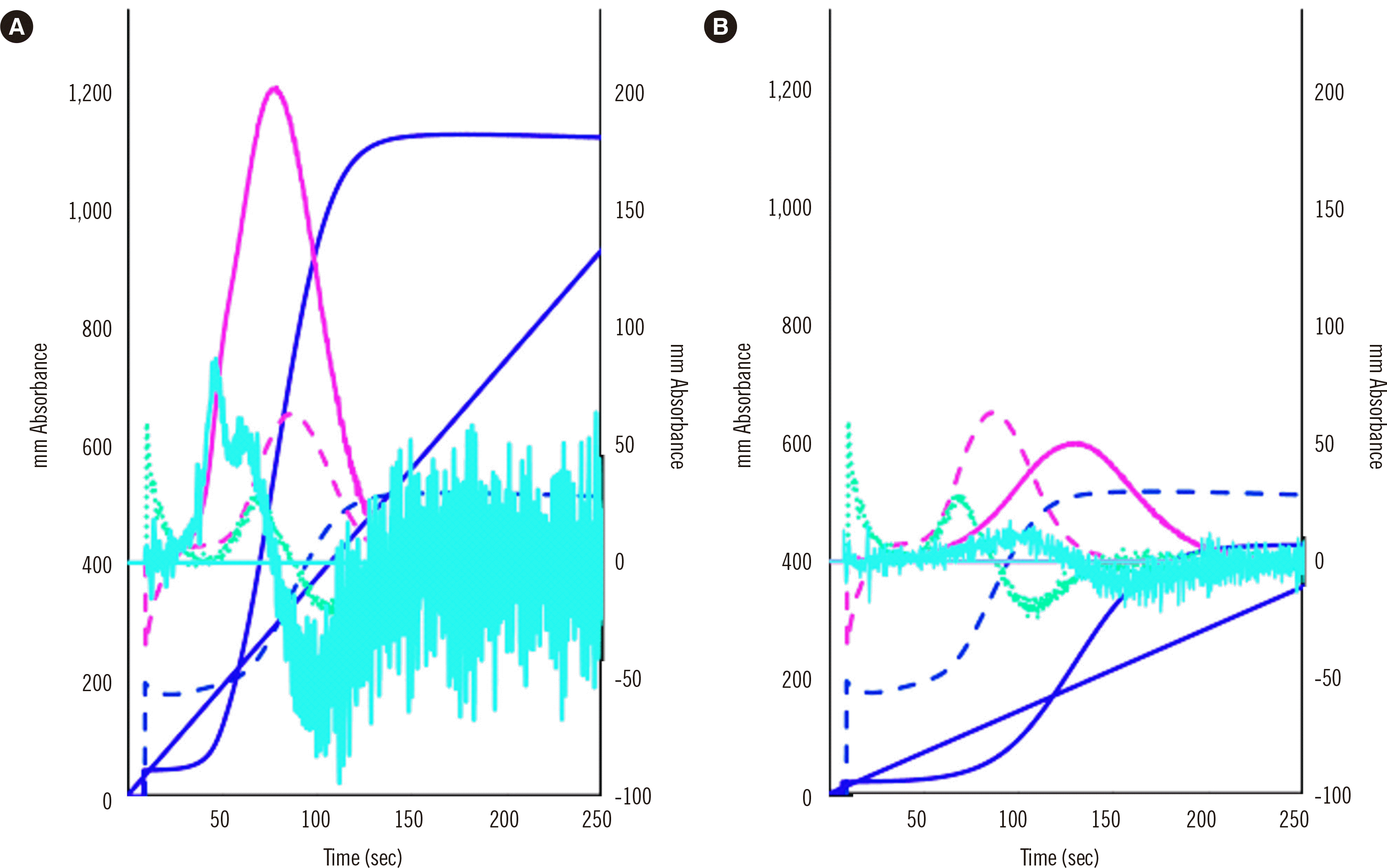
Fig. 5
CWA-TT using PPP (A and C) or PRP (B and D) from a healthy volunteer (A and B) and a patient with cancer (C and D). The fibrin formation curve is indicated in dark blue, the 1st DC (velocity) in pink, and the 2nd DC (acceleration) in light blue. The second peak of the 1st DC is significantly higher for PRP than for PPP and for the patient with cancer than for the healthy volunteer.
Abbreviations: CWA, clot waveform analysis; DC, derivative curve; TT, thrombin time; PPP, platelet-poor plasma; PRP, platelet-rich plasma.
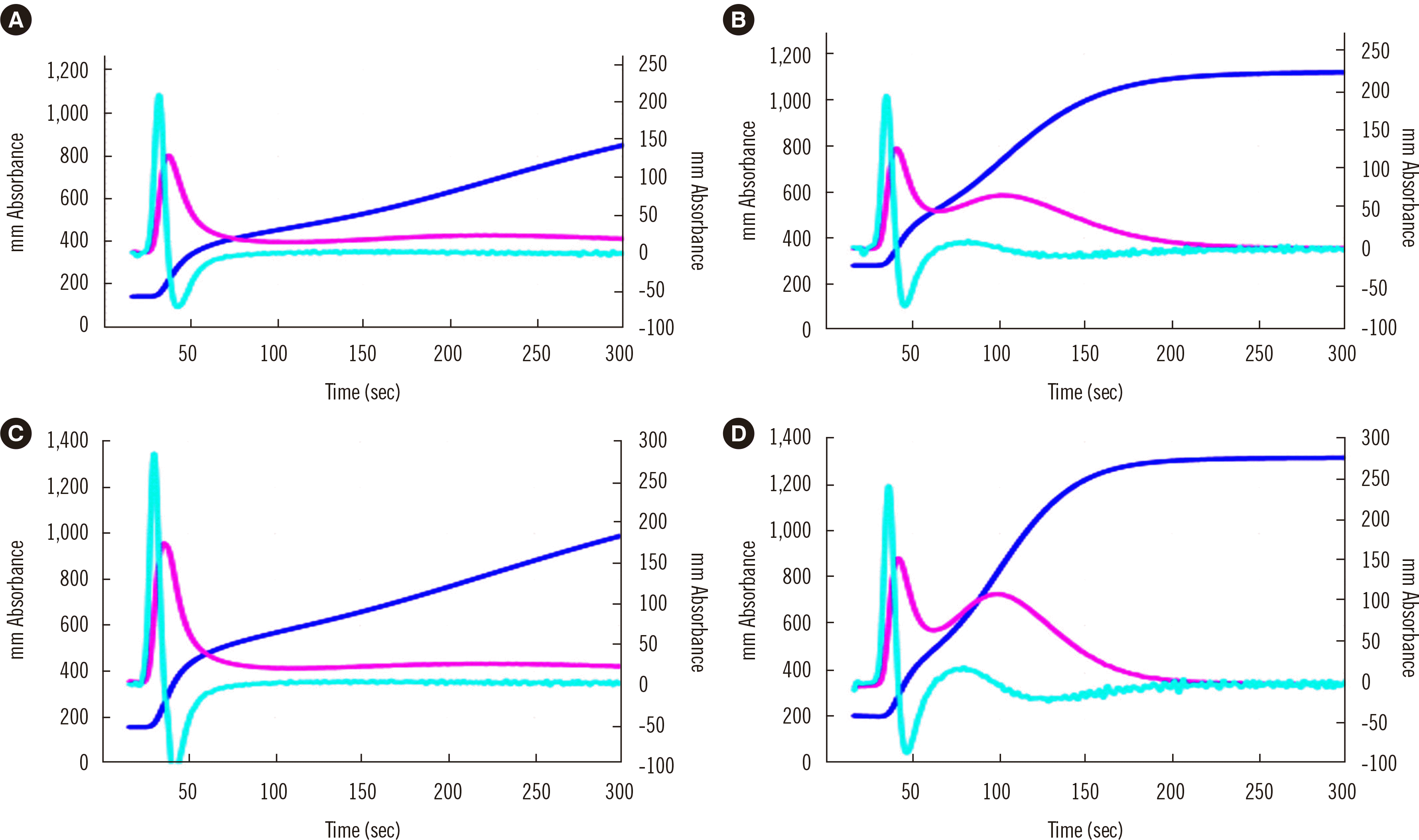
Fig. 6
Standard curves for the FVIII assay by CWA-TT and CWA-APTT in the presence or absence of Emi. The standard curve for CWA-TT is similar between Emi (+) and Emi (–), whereas that for CWA-APTT differs between Emi (+) and Emi (–).
Abbreviations: FFC, fibrin formation curve; PH, peak height; CWA, clot waveform analysis; TT, thrombin time; DC, derivative curve; PT, peak time; APTT, activated partial thromboplastin time; Emi, emicizumab.
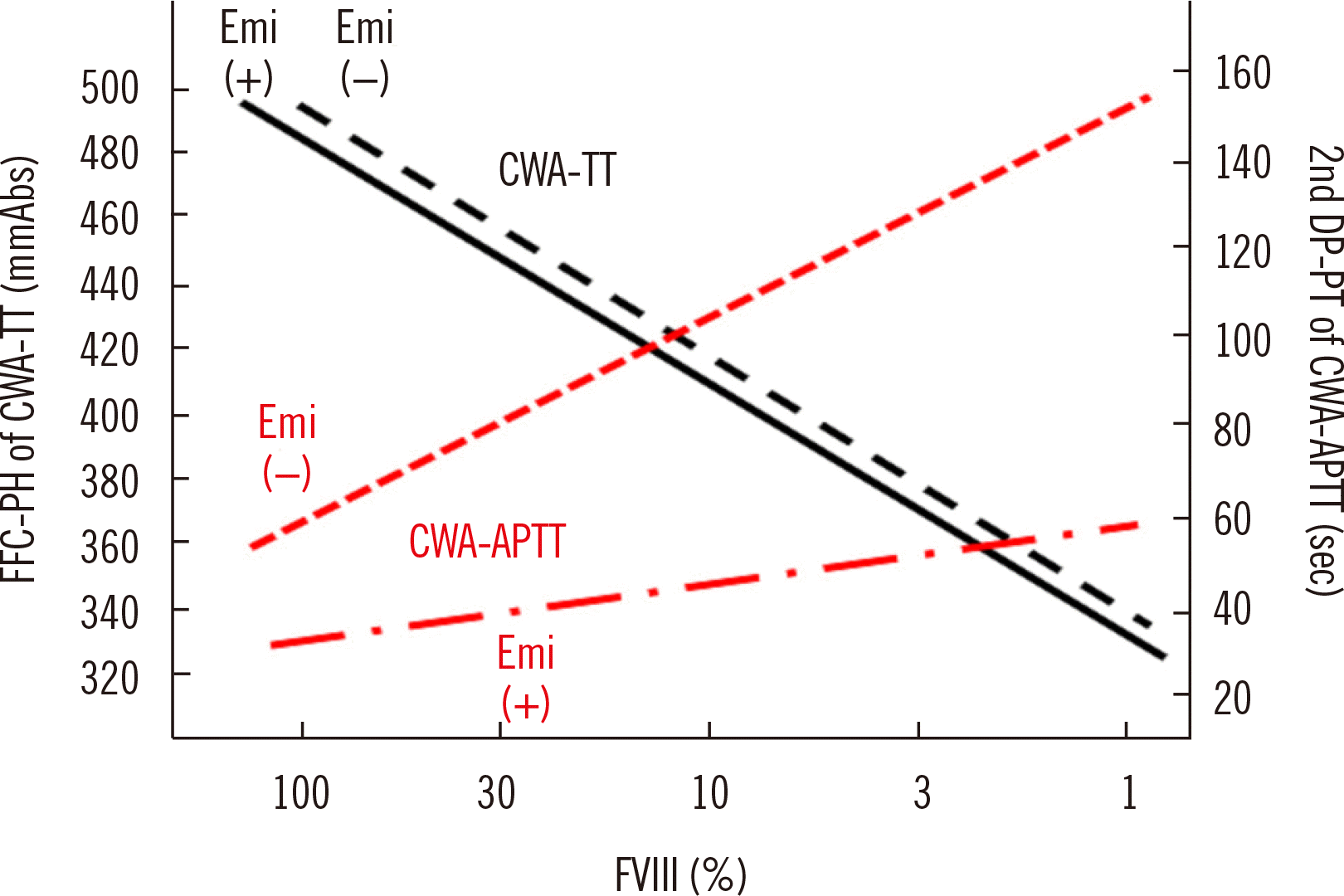
Table 1
Original and modified CWA
Abbreviations: CWA, clot waveform analysis; APTT, activated partial thromboplastin time; PT, prothrombin time; TT, thrombin time, dPT, dilute PT; dTT, dilute TT; PRP, platelet-rich plasma; sTF/FIXa, small amount of tissue factor-induced clotting factor IX activation; CFWA, clot-fibrinolysis waveform analysis
Table 2
Usefulness of CWA-APTT or sTF/FIXa for the evaluation of hemostatic abnormalities




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download