Abstract
Purpose
The aim of this study is to examine the effectiveness of the neutrophil-lymphocyte ratio (NLR) and CRP/albumin ratio (CAR) in evaluating disease severity and predicting clinical outcomes in patients diagnosed with acute cholecystitis (AC).
Methods
A total of 186 patients with AC were evaluated retrospectively. NLR, CAR, Mannheim Peritonitis Index (MPI), and P-POSSUM (Portsmouth Physiological and Operative Severity Score for the enUmeration of Mortality and Morbidity) scores were compared with AC severity grade.
Results
The rates of the grade 1 patients (group 1) and the grade 2–3 patients (group 2) were 57.5% (n = 107) and 42.5% (n = 79) according to the disease severity according to Tokyo Guidelines criteria (TG) 18/TG13, respectively. The morbidity rates determined in groups 1 and 2 were 26.7% (n = 28) and 51.9% (n = 41), respectively. No mortality was found in group 1, whereas the mortality rate in group 2 was 6.3% (n = 5). According to multivariate analysis, CAR (odds ratio [OR], 1.234; P < 0.001) and MPI (OR, 1.175; P = 0.001) were found to be associated with moderate-severe disease while CAR (OR, 1.109; P = 0.035) and P-POSSUM morbidity (OR, 1.063; P = 0.007) variables were found to be associated with the presence of morbidity.
Conclusion
We have demonstrated that CAR can be used in predicting severity of AC and that CAR is an alternative simple parameter of P-POSSUM morbidity score in prediction of morbidity in these cases. In addition to other assessment methods, these scores can provide valuable and complementary information in assessment of disease severity and prognosis in AC.
Hospital admissions due to acute cholecystitis (AC) are common, making it one of the most prevalent surgical disorders. AC ranks in the 6th place among gastrointestinal diseases in patients who are admitted to emergency service [1]. Differentiation between AC and cholelithiasis is important with respect to both emergency surgery as well as hospitalization in patients admitted to emergency and general surgery polyclinics with the complaint of right upper quadrant pain. Besides, accurate evaluation of disease severity is essential for treatment optimization, decision-making for emergency surgery, and achievement of a favorable prognosis [2]. The updated Tokyo Guidelines criteria (TG18/TG13) are the commonly used clinical, laboratory, and imaging evidence-based criteria in the diagnosis and clinical severity assessment of AC [3]. According to the TG18/TG13 criteria, grade 1 disease presents a mild-degree inflammation in the gallbladder, grade 2 involves moderate-degree inflammation without organ dysfunction, whereas a severe inflammation accompanied by organ dysfunction exists in grade 3. Emergency/early gall bladder drainage or urgent/early laparoscopic cholecystectomy in cases of medical treatment failure is recommended in grade 2 patients while emergency early gall bladder drainage is also suggested, as well as medical treatment and organ support in grade 3 patients [4]. Therefore, the accurate assessment of inflammation severity is essential in treatment management.
Numerous cancer types have shown a correlation between systemic inflammatory response and an unfavorable prognosis; and, accordingly, various inflammation-based prognostic scorings have been defined again in many cancer types, and prognostic value of these scores has been reported in various studies (neutrophil-lymphocyte ratio [NLR], CRP/albumin ratio [CAR], and Glasgow prognostic score) [56]. Additionally, it has been shown that these prognostic scores based on inflammation have been linked to the severity of the disease and outcomes in gastrointestinal disorders such as acute appendicitis, acute pancreatitis, ulcerative colitis, and Crohn disease [78].
Among these inflammatory scores, CAR is a novel parameter researched in recent times, and increased CAR value has been demonstrated to be associated with inflammation severity, poor prognosis, and mortality [910]. There is a limited number of studies that have addressed the relationship of CAR with disease severity and clinical outcomes in AC [11].
The other methods in the literature used to predict mortality and morbidity for inflammatory diseases and some cancer types are POSSUM (Physiological and Operative Severity Score for the enUmeration of Mortality and Morbidity) and P-POSSUM (Portsmouth POSSUM) scoring systems calculated based on evaluation of the physiological and surgical state of the patients [12].
The purpose of this study is to investigate the effectiveness of NLR and CAR as simple peripheral parameters in the assessment of disease severity and prediction of clinical outcomes (morbidities) in patients with AC.
The study protocol was approved by the Clinical Research Ethics Committee of University of Pamukkale Faculty of Medicine (No. 60116787-020/48514, date: August 18, 2020). The study was conducted in accordance with the 1964 principles of the Declaration of Helsinki and written informed consent was obtained from all the patients.
The hospital records of the patients who were treated with AC between January 2015 and December 2020 in the clinic of Department of General Surgery, Pamukkale University Faculty of Medicine were retrospectively evaluated. The demographic and clinical parameters of the patients, diagnostic radiological findings, treatment methods, and follow-up outcomes were reviewed from the hospital records. The patients with a history of congestive heart disease, hepatic and renal failure at diagnosis of hospital admission, ongoing use of anticoagulant and immunosuppressive drugs, history of malignancy, and the patients who received blood transfusion treatment and had a systemic infection within the last 4 weeks were excluded from the analysis.
The diagnosis of AC was confirmed using CT. TG18/TG13 criteria were used to group AC severity [3]. Moderate- (grade 2) and severe-degree (grade 3) inflammations were combined in a separate group as high-degree inflammation (grade 2–3) and further compared with mild-degree (grade 1) inflammation.
The clinical and demographic data involving age, gender, comorbid diseases, and treatment methods were obtained from the medical files. WBC count, neutrophil count, lymphocyte count, thrombocyte (platelet) count, CRP, international normalized ratio of PT, and hemoglobin levels along with AST, ALT, LDH, γ-GT, ALP, total bilirubin, direct bilirubin, creatinine, and albumin were assessed from blood samples taken at hospital admission and recorded.
NLR was calculated by dividing neutrophil count by lymphocyte count while CAR was calculated by dividing serum CRP level by serum albumin level.
The P-POSSUM risk scores of the patients were calculated at the website (www.riskprediction.org.uk) [13]. Mannheim Peritonitis Index (MPI) scores were calculated using the information in the patient files [14].
Management of the patients was made taking into consideration the general condition, comorbid diseases, and disease severity. All patients received medical therapy as initial treatment. Emergency gallbladder drainage or emergency cholecystectomy was performed in patients with insufficient treatment outcomes or treatment failure. The patients who recovered with medical therapy or had no contraindication were recommended elective cholecystectomy 6–8 weeks later and laparoscopic elective cholecystectomy was performed in the patients who accepted surgery.
Statistical analyses were performed using IBM SPSS Statistics ver. 25.0 (IBM Corp.). The continuous variables were presented as mean ± standard deviation. The categorical variables were demonstrated in terms of number and percentage. The Kruskal-Wallis test was used for the intergroup comparisons of continuous variables. The logistic regression analysis was used to evaluate the relationship of disease severity (grade) and presence of morbidity with laboratory parameters and odds ratio (OR) values with 95% confidence intervals were given in the table. The laboratory parameters that can be used in the receiver operating characteristic curve (ROC) analysis for prediction of moderate-severe grade disease and presence of morbidity were determined. The area under the ROC (AUC), cutoff, sensitivity, and specificity values were presented by the ROC analysis results. A P-value less than 0.05 was accepted as statistically significant.
Our study included 186 patients who were treated for AC. Of the patients, 83 (44.6%) were female and 103 (55.4%) were male with a mean age of 61 years (range, 17–94 years). No comorbidity was detected in 76 patients (40.9%) whereas hypertension, diabetes mellitus, ischemic heart disease (IHD), chronic renal failure, chronic obstructive pulmonary disease (COPD), comorbidity of hypertension and diabetes mellitus, and comorbidity of IHD and COPD were encountered in 28 (15.1%), 11 (5.9%), 10 (5.4%), 3 (1.6%), 1 (0.5%), 36 (19.4%), and 21 (11.3%), respectively.
Percutaneous transhepatic drainage was performed in 48 patients (25.8%) while 129 (66.2%) received medical therapy. No complication was experienced in 118 patients (63.8%) whereas hematoma, cholecystitis attack, cholangitis attack, pancreatitis attack, lesion site infection, biliary leakage, hemobilia, and gastrocutaneous fistula developed in 24 (12.9%), 17 (9.1%), 13 (7%), 1 (0.5%), 4 (2.2%), 3 (1.6%), 2 (1.1%), and 1 (0.5%), respectively. Five patients (2.7%) became exitus during hospital admission.
The rates of grade 1 patients and grade 2–3 patients were 57.5% (n = 107) and 42.5% (n = 79) according to the disease severity and TG18/TG13, respectively. The morbidity rates determined in grade 1 patients and grade 2–3 patients were 26.7% (n = 28) and 51.9% (n = 41), respectively. No mortality was found among grade 1 patients whereas the mortality rate in grade 2–3 patients was 6.3% (n = 5).
As can be seen in Table 1, a statistically significant difference between severity grades of AC in terms of demographic and clinical data such as age (P < 0.001), LDH (P = 0.002), ALP (P = 0.008), total bilirubin (P = 0.023), creatinine (P = 0.011), albumin (P < 0.001), WBC (P < 0.001), CRP (P < 0.001), CAR (P < 0.001), neutrophil (P < 0.001), lymphocyte (P = 0.048), NLR (P < 0.001), MPI (P < 0.001), P-POSSUM morbidity (P < 0.001), and P-POSSUM mortality (P < 0.001).
According to the evaluation of multivariate logistic regression analysis results in Table 2, the variables CAR (OR, 1.234, P < 0.001) and MPI (OR, 1.175, P = 0.001) were found significantly associated with moderate-severe disease (grade 2–3). Additionally, the variables CAR (OR, 1.109; P = 0.035) and P-POSSUM morbidity (OR, 1.063 P = 0.007) were determined to be significantly associated with the occurrence of morbidity.
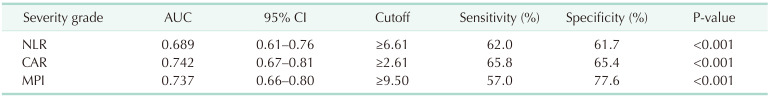
As shown detailed in Fig. 1 and Table 3, NLR, CRP/albumin, and MPI were found statistically significant regarding differentiation ability between grade 1 disease and grade 2–3 disease (P < 0.001, respectively). As shown in Fig. 2 and Table 4, the prediction of morbidity with CAR and P-POSSUM morbidity were determined to be statistically significant (P = 0.003 and P < 0.001, respectively). Aside from that, the predictive ability of P-POSSUM morbidity regarding the presence of morbidity was found higher than the other parameter (AUC, 0.670; sensitivity, 63.2%; specificity, 59.0%).
AC is one of the common causes among patients admitted to the emergency department and general surgery polyclinics with the complaint of abdominal pain [1]. Distention in the gallbladder due to obstruction of the cystic duct and inflammatory entities on the bladder wall are responsible for its pathophysiology and symptoms [15]. TG18/TG13 criteria are the clinical, laboratory, and imaging evidence-based criteria used in the diagnosis of AC and assessment of disease severity [3]. However, delays are still experienced in the diagnosis, more commonly in some patient groups (particularly in elderly patients and cases with comorbidities), despite all the improvements in diagnostic and therapeutic methods in the present time [1617]. Particularly, more commonly in these patient groups, disease progression and serious complications such as gangrenous cholecystitis, abscess, and perforation may develop; consequently, morbidity and mortality rates may increase [1718]. Therefore, assessment of inflammation severity has critical importance in the determination of treatment approach and prognosis [3419]. Recently, inflammatory indicators have been shown to be associated with disease severity and prognosis in many cancer types and inflammatory diseases, and there is still a need for novel parameters on that subject [581020]. In the present study, NLR and CAR, which are the simple peripheral blood parameters, were evaluated as inflammatory markers in the assessment of disease severity and prediction of morbidity in patients with AC. Multivariate analysis revealed that CAR was independently associated with the severity of the disease and effective in the prediction of morbidity.
The mean age was 61 years and 55.4% of the patients were male in our study consistent with the literature [21]. Also, in a study by Gökçe and Gökçe [22], the rate of male gender was higher than female gender in the patients with AC, similar to our study.
The assessment of disease severity grade is important for both treatment planning and the determination of patient prognosis in patients with AC. It has been demonstrated in a recent study that disease severity grade assessed according to TG13 criteria is an independent predictor of mortality in patients with AC [19]. Likewise, it has been reported in the studies carried out in recent years that there is a significant relationship between disease severity grade based on TG13 criteria and 30-day mortality rates [211]. Also in our study, disease severity has been graded according to updated TG18/TG13 criteria, consistent with the literature, and no mortality was encountered in grade 1 disease whereas 57.1% mortality was detected in grade 3 disease.
It has been shown in many studies that inflammation-based prognostic markers have a prognostic value for many cancer types [67]. It has been reported that these inflammation-based markers may be an indicator in the prediction of disease severity and outcomes of also various inflammatory diseases in the gastrointestinal tract such as acute appendicitis and chronic inflammatory bowel disorders. It has been manifested that high NLR values are related to gangrenous appendicitis, hospitalization duration, and the risk of surgical complications in patients with acute appendicitis [823]. The study by Qin et al. [9] exhibited the benefit of CAR in the assessment of disease activity in Crohn disease while Kim et al. [24] reported in their study that CAR is an independent predictor for mortality in sepsis patients. In the literature, some studies have evaluated WBC and CRP as the inflammatory parameters in patients with AC [2526]. High WBC and CRP values were found to be associated with severe forms of AC and particularly high CRP level has been detected to be a predictor of gangrenous cholecystitis [25]. Beliaev et al. [26] have presented in a large series of 1,843 cases that a high level of CRP is a useful marker in the diagnosis and disease severity assessment of AC, and CRP value was found superior to WBC in the diagnosis of AC in their study.
Also in our study, consistent with the literature, there is an association between simple peripheral inflammatory markers and disease severity grade [1120]. Beliaev et al. [20] reported in their study that NLR was a more useful marker than WBC in the assessment of disease severity for AC, disease severity was determined by histopathological findings in that study. On the other side, disease severity grade was identified based on TG criteria in the study by Sato et al. [11], similar to our study. Similar to our outcomes, it has been shown by multivariate analyses in their study that CAR was associated with grade 2–3 AC as well as modified Glasgow Prognostic Score as another inflammatory marker, this outcome was explained by the fact that a high WBC value is one of the diagnostic criteria of grade 2 AC. In our study, the univariate ROC analysis results suggested that NLR, CAR, and MPI were statistically significant in the prediction of moderate and severe grades. However, multivariate logistic regression analyses revealed only CAR and MPI to be associated with the grade of the disease. The association of MPI with disease severity in patients with peritonitis has become more prominent in previous studies regarding the prediction of [27]. Our findings indicate that CAR values are more important than NLR. Additionally, CAR along with P-POSSUM morbidity index may play a complementary role in the prediction of morbidity.
The decreased albumin synthesis as a result of the hypercatabolic state due to the inflammatory process and downregulation of cytokines such as tumor necrosis factor-alpha and interleukin-6 is inversely proportional with the grade of the inflammatory response [28]. Additionally, albumin level is also associated with nutritional state [27]. High CRP and low albumin levels may be indicators of severe inflammation. Hypercytokinemia due to chronic and severe inflammation leads to inadequate nutrition and weight loss [29]. CAR shows the combination of systemic inflammation and nutritional state are used in the prediction of patient outcomes in many diseases such as sepsis, acute pancreatitis, activation of ulcerative colitis, and hepatocellular and pancreatic cancer [2930]. In our study, simple peripheral blood parameters, MPI, and P-POSSUM scores were evaluated as parameters in the prediction of morbidity. CAR and P-POSSUM morbidity scores were determined to be statistically significant by multivariate logistic regression analyses in the prediction of morbidity.
The retrospective design of our study and the small number of patients particularly in the grade 3 AC group were the limitations of our study. However, we conclude that the use of scoring systems in which efficacies have been accepted in general surgery practice from past to present time, as well as simple peripheral blood parameters, constituted the strong side of the study.
We have demonstrated that CAR and MPI are independently associated with the severity of AC. CAR, which is a simple parameter, can also be used as an alternative to P-POSSUM morbidity score in the prediction of morbidity in these patients. These scores may play a complementary role in the assessment of disease severity and prognosis in AC.
References
1. Peery AF, Crockett SD, Barritt AS, Dellon ES, Eluri S, Gangarosa LM, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology. 2015; 149:1731–1741. PMID: 26327134.
2. Yokoe M, Takada T, Hwang TL, Endo I, Akazawa K, Miura F, et al. Validation of TG13 severity grading in acute cholecystitis: Japan-Taiwan collaborative study for acute cholecystitis. J Hepatobiliary Pancreat Sci. 2017; 24:338–345. PMID: 28419779.
3. Yokoe M, Hata J, Takada T, Strasberg SM, Asbun HJ, Wakabayashi G, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018; 25:41–54. PMID: 29032636.
4. Okamoto K, Suzuki K, Takada T, Strasberg SM, Asbun HJ, Endo I, et al. Tokyo Guidelines 2018: flowchart for the management of acute cholecystitis. J Hepatobiliary Pancreat Sci. 2018; 25:55–72. PMID: 29045062.
5. Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008; 32:1757–1762. PMID: 18340479.
6. McMillan DC. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc. 2008; 67:257–262. PMID: 18452641.
7. Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients withhepatocellular carcinoma. Ann Surg Oncol. 2015; 22:803–810. PMID: 25190127.
8. Shimizu T, Ishizuka M, Kubota K. A lower neutrophil to lymphocyte ratio is closely associated with catarrhal appendicitis versus severe appendicitis. Surg Today. 2016; 46:84–89. PMID: 25686778.
9. Qin G, Tu J, Liu L, Luo L, Wu J, Tao L, et al. Serum albumin and C-reactive protein/albumin ratio are useful biomarkers of Crohn’s disease activity. Med Sci Monit. 2016; 22:4393–4400. PMID: 27848931.
10. Ranzani OT, Zampieri FG, Forte DN, Azevedo LC, Park M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS One. 2013; 8:e59321. PMID: 23555017.
11. Sato N, Kinoshita A, Imai N, Akasu T, Yokota T, Iwaku A, et al. Inflammation-based prognostic scores predict disease severity in patients with acute cholecystitis. Eur J Gastroenterol Hepatol. 2018; 30:484–489. PMID: 29303882.
12. Prytherch DR, Whiteley MS, Higgins B, Weaver PC, Prout WG, Powell SJ. POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity. Br J Surg. 1998; 85:1217–1220. PMID: 9752863.
13. Jason Smith-Consultant Surgeon. P-POSSUM Scoring [Internet]. Jason Smith;c2023. cited 2021 May 19. Available from: www.riskprediction.org.uk. http://www.riskprediction.org.uk/index-pp.php
.
14. Linder MM, Wacha H, Feldmann U, Wesch G, Streifensand RA, Gundlach E. [The Mannheim peritonitis index. An instrument for the intraoperative prognosis of peritonitis]. Chirurg. 1987; 58:84–92. PMID: 3568820.
15. Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. Br J Surg. 2004; 91:734–738. PMID: 15164444.
16. Parker LJ, Vukov LF, Wollan PC. Emergency department evaluation of geriatric patients with acute cholecystitis. Acad Emerg Med. 1997; 4:51–55. PMID: 9110012.
17. Papadakis M, Ambe PC, Zirngibl H. Critically ill patients with acute cholecystitis are at increased risk for extensive gallbladder inflammation. World J Emerg Surg. 2015; 10:59. PMID: 26628907.
18. Fagan SP, Awad SS, Rahwan K, Hira K, Aoki N, Itani KM, et al. Prognostic factors for the development of gangrenous cholecystitis. Am J Surg. 2003; 186:481–485. PMID: 14599611.
19. González-Muñoz JI, Franch-Arcas G, Angoso-Clavijo M, Sánchez-Hernández M, García-Plaza A, Caraballo-Angeli M, et al. Risk-adjusted treatment selection and outcome of patients with acute cholecystitis. Langenbecks Arch Surg. 2017; 402:607–614. PMID: 27704274.
20. Beliaev AM, Angelo N, Booth M, Bergin C. Evaluation of neutrophil-to-lymphocyte ratio as a potential biomarker for acute cholecystitis. J Surg Res. 2017; 209:93–101. PMID: 28032577.
21. Pehlivan T, Alper Cevik A, Ateş E. [Relationships among ultrasonographic and demographic, clinical, laboratory findings of patients with acute cholecystitis]. Ulus Travma Acil Cerrahi Derg. 2005; 11:134–140. PMID: 15877244.
22. Gökçe FS, Gökçe AH. Is C-reactive protein a superior marker of inf lammation over the neutrophil/lymphocyte ratio or platelet/lymphocyte ratio in acute cholecystitis? Dicle Med J. 2019; 46:839–845.
23. Kelly ME, Khan A, Riaz M, Bolger JC, Bennani F, Khan W, et al. The utility of neutrophil-to-lymphocyte ratio as a severity predictor of acute appendicitis, length of hospital stay and postoperative complication rates. Dig Surg. 2015; 32:459–463. PMID: 26488396.
24. Kim MH, Ahn JY, Song JE, Choi H, Ann HW, Kim JK, et al. Correction: the C-reactive protein/albumin ratio as an independent predictor of mortality in patients with severe sepsis or septic shock treated with early goal-directed therapy. PLoS One. 2019; 14:e0225620. PMID: 31738808.
25. Mok KW, Reddy R, Wood F, Turner P, Ward JB, Pursnani KG, et al. Is C-reactive protein a useful adjunct in selecting patients for emergency cholecystectomy by predicting severe/gangrenous cholecystitis? Int J Surg. 2014; 12:649–653. PMID: 24856179.
26. Beliaev AM, Marshall RJ, Booth M. C-reactive protein has a better discriminat ive power than white cell count in the diagnosis of acute cholecystitis. J Surg Res. 2015; 198:66–72. PMID: 26038247.
27. Sökmen S, Coker A, Unek T, Tunçyürek P, Bora . [Effectiveness of the Mannheim Peritonitis Index in patients with peritonitis]. Ulus Travma Derg. 2001; 7:100–103. PMID: 11705031.
28. Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol. 2005; 39(4 Suppl 2):S143–S146. PMID: 15758650.
29. Tominaga T, Nonaka T, Sumida Y, Hidaka S, Sawai T, Nagayasu T. The C-reactive protein to albumin ratio as a predictor of severe side effects of adjuvant chemotherapy in stage III colorectal cancer patients. PLoS One. 2016; 11:e0167967. PMID: 27930703.
30. Kaplan M, Ates I, Akpinar MY, Yuksel M, Kuzu UB, Kacar S, et al. Predictive value of C-reactive protein/albumin ratio in acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2017; 16:424–430. PMID: 28823374.
Fig. 1
A comparison regarding the area under the receiver operating characteristic (ROC) curve to assess the differentiation between grade 1 and grade 2–3 disease. NLR, neutrophil-lymphocyte ratio; CAR, CRP/albumin ratio; MPI, Mannheim Peritonitis Index.
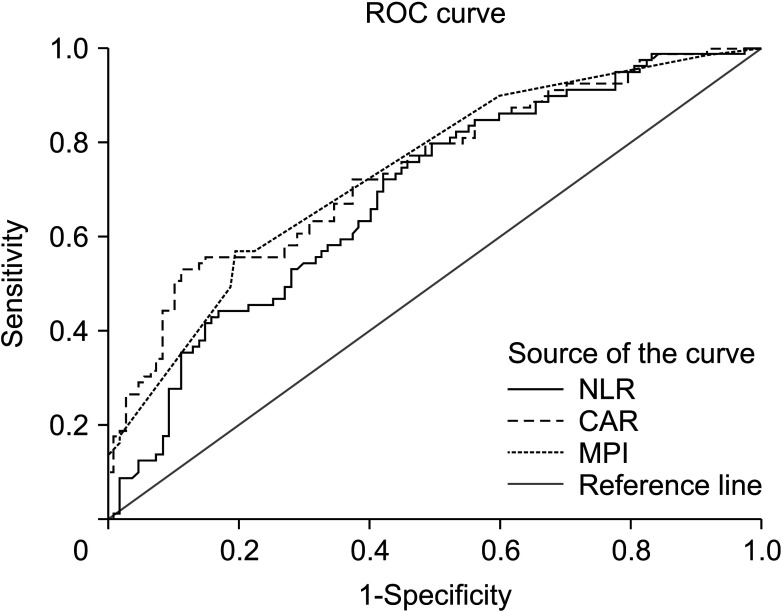
Fig. 2
A comparison regarding the area under the receiver operating characteristic (ROC) curve to assess the prediction of morbidity. CAR, CRP/albumin ratio; P-POSSUM, Portsmouth Physiological and Operative Severity Score for the enUmeration of Mortality and Morbidity.

Table 1
The comparison between the demographic and clinical data of the patients grouped according to disease severity
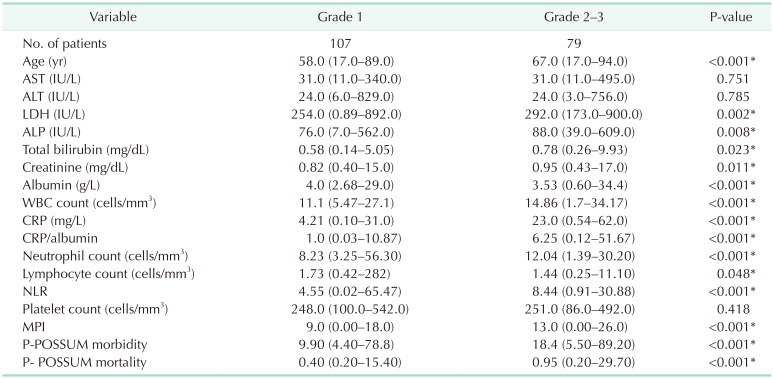
Table 2
The analysis of predictor factors for moderate and severe disease (grade 2–3) and occurrence of morbidity
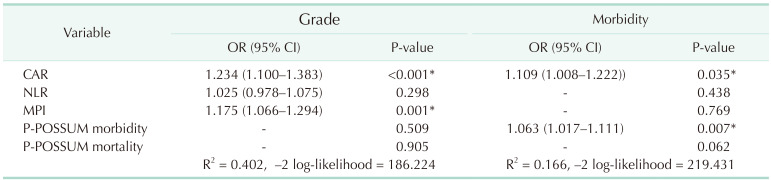
Values are presented as number only or mean (range).
OR, odds ratio; CI, confidence interval; CAR, CRP/albumin ratio; NLR, neutrophil-lymphocyte ratio; MPI, Mannheim Peritonitis Index; P-POSSUM, Portsmouth Physiological and Operative Severity Score for the enUmeration of Mortality and Morbidity.
*P < 0.05, statistically significant.
Table 3
A comparison regarding the area under the curve to assess the differentiation between grade 1 and grade 2–3 disease




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download