A 7-year-old girl with ESKD on PD was admitted due to fever, malaise, and poor oral intake on December 31, 2021. An intermittent fever (37.8–38.5 ℃) persisted for 2 weeks despite oral antibiotic treatment (cefditoren 3 mg/kg/day) in the outpatient clinic. She was born at gestational age 33 weeks 6 days, weighing 4,120 g. She was admitted to the neonatal intensive care unit owing to a prenatally identified large sacrococcygeal immature teratoma (size 17×14×7 cm) and multiple anomalies, including rectal atresia and fixed equinus of both feet. Excisional operation of the teratoma was performed on the eighth day after birth. She developed ESKD due to teratoma-induced renal vessel compression and ischemic events with cardiac arrest during the operation, which was treated with continuous kidney replacement therapy for 3 months, followed by chronic continuous PD. She was discharged at 5 months at 3.9 kg body weight with a PD modality consisting of eight dialysis cycles with 60 mL of 2.5% dextrose dialysate at 2.5-hour intervals. As she grew up, she adapted to an automated continuous-cyclic PD schedule: 350 mL of 1.5% dextrose dialysate exchanges for six cycles at 2-hour intervals from 8 PM to 8 AM, followed by 300 mL of 7.5% glucose dialysate retention for 12 hours. She had suffered from several infectious PD complications, including peritonitis, exit site infections, and wound dehiscence and underwent three rounds of PD catheter change operations and two rounds of laparoscopic exploration and peritoneal adhesiolysis operations. The most recent case of peritonitis due to
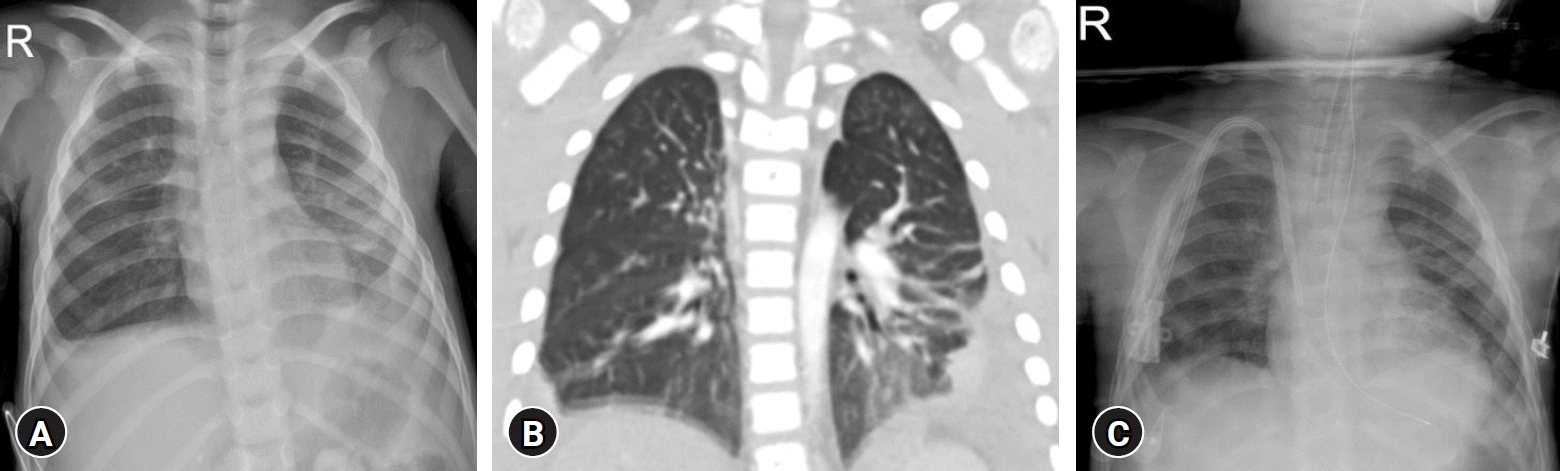
klebsiella aerogenes occurred 2 months before admission. On admission, she had symptoms including fever, chill, general weakness, weight loss of 1 kg over 1 month, intermittent cough, and rhinorrhea. She had no signs of respiratory distress, such as tachypnea, dyspnea, and cyanosis. Her height was 93.3 cm (<3rd percentile), and her weight was 11.6 kg (<3rd percentile). Her vital signs were as follows: systolic/diastolic blood pressure 68/38 mmHg, heart rate 123/min, respiratory rate 20/min, body temperature 37.8 ℃, and oxygen saturation 95% in room air. On physical examination, she appeared chronically ill. Lung sounds were clear without wheezing or crackles. Bowel sounds were normoactive, with a soft abdomen without focal abdominal tenderness. The vesicostomy and ileostomy (due to rectal atresia) sites were clear. Pitting edema was not observed. Initial laboratory evaluation was as follows: white blood cell counts 26,600/μL (neutrophils 82.7%, lymphocytes 8.5%, monocytes 6.1%), hemoglobin 9.2 g/dL, platelets 365,000/μL, blood urea nitrogen 50 mg/dL, creatinine 8.23 mg/dL, B-type natriuretic peptide 2 pg/mL (normal range: <36 pg/mL), and C-reactive protein (CRP) 7.44 mg/dL (normal range: 0–0.6 mg/dL). PD fluid analysis was clear with a differential count of nucleated cells 82/μL consisting of 41% neutrophils and 15% lymphocytes. Chest radiography and computed tomography (CT) revealed multifocal peribronchial infiltration with subpleural consolidation in both lower lungs and suggestive complicated bilateral pleural effusion with diffuse enhancing pleural thickening (
Fig. 1). Multiple reactive lymph node hyperplasias were also observed in the mediastinum, lobar/interlobar, and axillae. Left ureteral residual contrast dye from voiding cystourethrography, performed 80 days before admission, was observed on X-rays. Abdominopelvic CT results were as follows: PD catheter insertion with its tip in the pelvic cavity, moderate ascites with mild peritoneal thickening in the upper abdomen, bilateral hydronephroureterosis, a severe parenchymal atrophic change in both kidneys, residual contrast material in the left pelvicalyceal system, and irregular lobulated margined bladder. Considering her pneumonic features, we initially treated her with ampicillin-sulbactam (50 mg/kg/day ampicillin, 30 mg/kg/day/sulbactam). However, we amended to piperacillin-tazobactam (100 mg/kg/day piperacillin, 25 mg/kg/day tazobactam) on the seventh hospital day to cover
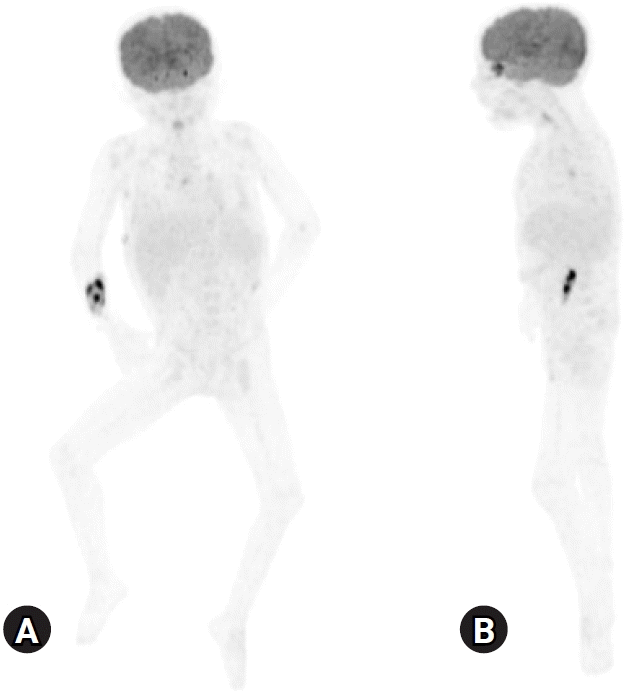
K. aerogenes, which was the cause of peritonitis 2 months before. Nevertheless, her CRP increased to 11.7 mg/dL with persistent mild fever (<38.0 ℃). We subsequently switched to vancomycin (10 mg/kg/day) and meropenem (20 mg/kg/day) combination therapy on hospital day 13, and initial and repeated culture studies, including blood, PD fluid, sputum, and stool, were all negative. Tests for severe acute respiratory syndrome coronavirus 2, other respiratory viruses, tuberculosis interferon-gamma, and mycoplasma antibodies were also negative. We had performed a cystoscopy to remove contrast media of the left pelvicalyceal system injected during the voiding cystourethrography study for the transplantation work up 4 months before, considering the possibility of contrast-induced inflammation; however, the patient’s fever elevated to 40.2 ℃ and CRP to 13.3 mg/dL after the procedure. The culture result of the drained contrast was negative. We also performed whole body positron emission tomography-CT (
18F-fludeoxyglucose) (
Fig. 2). Despite observing mild hypermetabolic uptake in both lungs and pleural effusion, ascites, and lymph nodes in the right lower paratracheal, subcarinal, pulmonary hilar, and left inguinal areas, we concluded that the possibility of malignancy was low. Although we requested that a radiologist and a thoracic surgeon drain the pleural effusion to identify any undiscovered organisms or malignancies that might have caused prolonged inflammation, they could not aspirate or drain the effusion as the absolute amount of fluid was too small. We discontinued vancomycin first to rule out drug fever after 4 weeks of treatment. The intermittent fever occurred up to 38.3 ℃ but then dropped, and CRP decreased to 5.63 mg/dL. Nine days later, intravenous meropenem treatment was converted to intraperitoneal imipenem administration to cover potentially undetected pleural or peritoneal
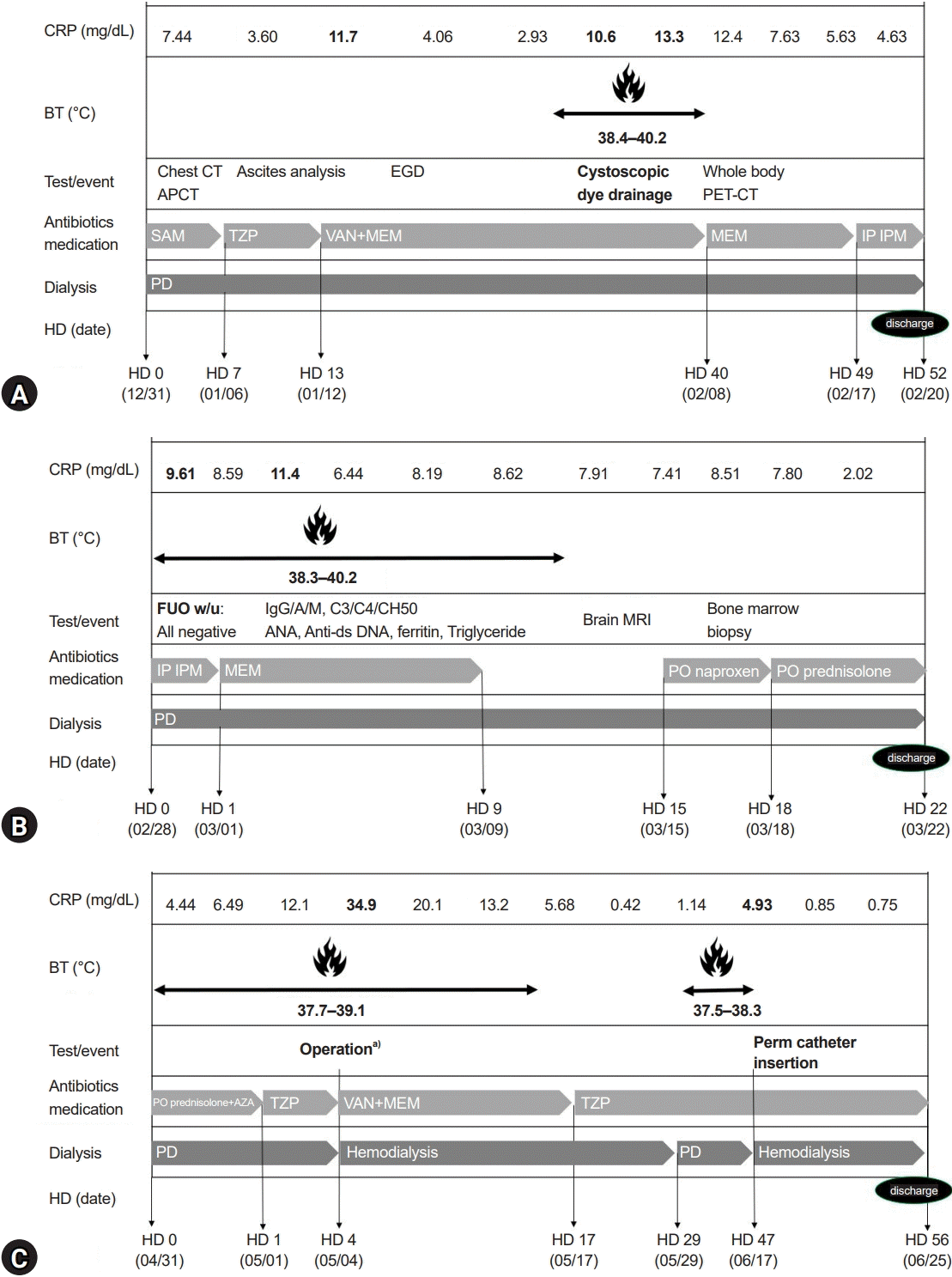
K. aerogenes infection. The fever disappeared, CRP further decreased to 4.61 mg/dL, and the patient was discharged after 52 days of admission (
Fig. 3A). She was re-admitted a week later due to re-aggravated fever and CRP of 40.2 ℃ and 9.61 mg/dL, respectively. Intraperitoneal imipenem treatment was changed to intravenous meropenem. A total of 12 times PD fluid bacteria/fungus/acid-fast bacilli culture tests performed for 3 months were all negative, and white blood cell counts of PD fluid analyses were normal (
Table 1). We discontinued antibiotics 9 days after readmission, as we could not identify any infection focus and had to differentiate drug fever. We performed a follow-up CT of the chest and abdomen, indicating findings similar to previous studies. We evaluated the fever of unknown origin (FUO), including immunologic workups, brain magnetic resonance images, and bone marrow biopsy, and the results were nonspecific. We diagnosed her with systemic juvenile idiopathic arthritis as we could not find any other cause (
Table 2), started naproxen on hospital day 15, and subsequently added prednisolone (2 mg/kg/day) on day 18. The fever disappeared, and CRP dropped to 2.02 mg/dL. She was discharged after 22 days of admission (
Fig. 3B), and azathioprine (1–2 mg/kg/day) was added at the outpatient clinic with CRP ranging from 2.16 to 4.44 mg/dL. She was admitted for bladder augmentation before kidney transplantation on May 1, 2022. We discontinued immunosuppressants, added stress-dose hydrocortisone, and started prophylactic antibiotics (piperacillin-tazobactam) before surgery. Bladder augmentation and left nephroureterectomy operation were electively performed on hospital day 4. After open abdominal surgery, we stopped her PD and started hemodialysis. CRP elevated to 34.9 mg/dL 2 days after surgery with a mild fever (<38.0 ℃). We, therefore, switched from piperacillin-tazobactam to vancomycin and meropenem. CRP slowly decreased without fever while the patient was on hemodialysis for 2 weeks instead of PD. CRP was normalized to 0.42 mg/dL for the first time within 6 months after initial fever onset. A small amount of pleural effusion was still observed on chest X-rays. Three weeks after surgery, after restarting PD, a mild fever developed (37.9 ℃), and CRP elevated to 4.93 mg/dL. We planned to perform peritoneal scintigraphy to identify the presence of a PPC, but the test was not available for children at our center. We requested a thoracic surgeon to explore the diaphragm to find a fistula or foramen; however, he refused the procedure because pleural thickening had already progressed, and diaphragm detachment was expected to be very difficult. We ceased PD and restarted hemodialysis, which normalized body temperature, and CRP dropped to 0.78 mg/dL. After resuming PD to confirm if PD and the PPC were the cause of the fever and elevation of inflammatory markers, body temperature rose and CRP increased. We finally decided to stop PD to switch to hemodialysis. The PD catheter was removed, the right subclavian perm catheter was inserted on June 17, 2022, and hemodialysis was stably started and maintained at a 3 times/week schedule (
Fig. 3C). Since she started hemodialysis, her fever has not recurred, and pleural effusion, pneumonia, and inflammation markers have slowly improved. The FUO that persisted for 6 months, despite treatment with broad-spectrum antibiotics and immunosuppressants, was finally resolved. The bilateral pleural effusion also gradually improved after discontinuing PD (
Fig. 1C). Currently, she attends outpatient clinics and adapts well to hemodialysis, which is scheduled 3 times a week, while awaiting kidney transplantation.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download