Abstract
Undeniably, endothelial cells (EC) contribute to the maintenance of the homeostasis of the organism through modulating cellular physiology, including signaling pathways, through the release of highly active molecules as well as the response to a myriad of extrinsic and intrinsic signaling factors. Review the data from the current literature on the EC role in norm and disease. Endothelium maintains a precise balance between the released molecules, where EC dysfunction arises when the endothelium actions shift toward vasoconstriction, the proinflammatory, prothrombic properties after the alteration of nitric oxide (NO) production and oxidative stress. The functions of the EC are regulated by the negative/positive feedback from the organism, through EC surface receptors, and the crosstalk between NO, adrenergic receptors, and oxidative stress. More than a hundred substances can interact with EC. The EC dysfunction is a hallmark in the emergence and progression of vascular-related pathologies. The paper concisely reviews recent advances in EC (patho) physiology. Grasping EC physiology is crucial to gauge their potential clinical utility and optimize the current therapies as well as to establish novel nanotherapeutic molecular targets include; endothelial receptors, cell adhesion molecules, integrins, signaling pathways, enzymes; peptidases.
Tremendous advances have been made in vascular biology in recent years. Physiologically, the endothelium comprises highly specialized cells that line the blood and lymphatic vessels, weigh 1 kg and build approximately 400 m2 in an adult person that maintains approximately 60,000 miles of blood vessels. Endothelial cells (EC) performs a wide range of crucial metabolic homeostatic activities to maintain a balance between aggression and protection factors by responding to extrinsic and intrinsic signals and releasing specific active molecules, as well as remodeling signaling pathways [1-3]. Firstly identified molecules were antithrombosis and hemostatic agents (protein c and s, thrombomodulin [TM], calmodulin [CaM], tissue-type plasminogen activator, plasminogen activator inhibitor type I, platelet activating factor, von Willebrand factor [vWF] that stay in Weibel–Palade bodies), pro and anti-inflammatory molecules (E-selectin, P-selectin, ICAM-1, VCAM-1, PECAM-1, Decay Acceleration Factor, CD59), and vasoactive substances (endothelin 1 [ET 1], thromboxane A2, superoxide, prostaglandin F2a, prostacyclin I2, endothelium-derived hyperpolarising factor, nitric oxide [NO], urotensin, C type natriuretic peptide [CNP], adrenomedullin, adenosine, purines, reactive oxygen species, H2S, angiotensin II, bradykinin) [4-8]. A couple of these molecules are released under the effect of extrinsic factors (cytokines) such as urokinase-type plasminogen activator. Interestingly, the EC release many molecules and simultaneously express receptors for them, which makes the endothelium behave like autocrine cells as well as paracrine. The self-regulation mechanisms various from organ to other, according to the requirement and the nature of the presented tissue, therefore the bearing capabilities differ in each organ and this probably shows the susceptibility of endothelial dysfunction variation in different tissues and why usually endothelial dysfunction is characterized for some tissues and not for others [9]. On the other hand, these distinct morphophysiological variations in the EC are probably can be used to enhance the selectivity of the endothelium-delivered drug. For example, EC in the branching points express different genes than other areas [10]. Besides, caveolae are extensively expressed in cardiac and pulmonary EC, whereas caveolae are absent in cerebral EC. Also, endothelial cell protein C receptor (EPCR) is enriched in large vessels and has low expression in the smaller vessels [8].
Furthermore, EC can modify their metabolic activities, probably, through modulation of active substance releasing, according to the triggered endothelial plasma membrane receptor [11-13]. Usually, the transient receptor potential (TRP) and G-coupled-protein receptor play a leading role in triggering the intracellular signaling cascade, later activate specific Ca+2 channels or K+ channels as well as probably promote specific gene expression or repressions such as pro-inflammatory genes that encode for cytokines, via activating the nuclear factor-κB or mitogen-activated protein kinase pathways [14, 15]. Moreover, the EC receptors perform recognition and sensation activity for IL6, IL8, H2S, TNF-related activation protein; E-selectin, P-selectin, ICAM-1, VCAM-1, PECAM-1; D-Dimer, vWF, TF, PAT-1; iNOS, nitrotyrosine; PECAM-1, MadCAM-1, JAM-1, Cav-1; CECs, EMPs; CRP, PTX3 via sensor systems including glycocalyx, caveolae, cytoskeleton and integrins interconnected with components of extracellular matrix and intercellular junctions; in particular, PECAM-1 (Table 1) [5, 16]. The G-coupled-protein receptor and Src-dependent tyrosine kinase, endothelial nitric oxide synthetase (eNOS), or ion channel signaling pathway of the EC are strictly related to the caveolin-1, micro-particle protein embedded in an omega-shaped cholesterol and glycosphingolipid rich caveolae, 15% of the EC membrane [6]. The topography of the EC gives them a critical physiological and pathological role in the progression of the diseases since EC sense all changes in the organism via direct interaction with the blood biomarkers of the disease. Therefore, the endothelium plays a role in the promotion of pathogenesis. Indeed, many of these biomarkers are of inflammatory response to protect against the invaders but unfortunately, usually, accompanied by self-damage. However, normally, the advantages weigh more than self-damage. It is extremely important that the endothelium is the executive representative of the cardiopulmonary system on the tissue side.
The paper aimed to analyze the recent advances in the role of blood EC in physiopathology and prescribe these functions; namely, control of vascular permeability, adhesiveness, contractility, and formation of new vessels (angiogenesis), detection of mechanical forces related to blood flow and vessel contraction, the interaction of blood components with vessels and underlying tissues, blood metabolism, clotting and fluidity, and gas exchange.
ET 1 is a high-yield vasoactive substance that is primarily synthesized by the EC of the blood and lymphatic trees [28]. However, ET 1 effects are not limited to the vascular tree 2 and have a high affinity to ETA, ETB, and ETC receptors, >60% of endothelin heart receptors are ETA and vice versa in the lung, liver, and kidney, predominant ETB [29]. The presence of various isotypes of endothelin receptors makes ET 1 a universal active substance, ET 1 receptors are present in the cardiovascular system (endothelium, VSMCs, cardiomyocytes), urinary system (renal medulla), nervous system (neurons), immune system and skin (macrophages, leukocytes, mast cells, Kupffer cells), respiratory system (tracheal epithelium, airway epithelial cells), other tissues and cells (fibroblasts, hepatic sinusoids) [30, 31]. Physiologically, four types of endothelin receptors are present in the organism (ETA, ETB1, ETB2, ETC); however, only two of them have been well studied. In fact, endothelial-endothelin production is enhanced by the activation of activator protein-1, GATA-2, smad, hypoxia-inducible factor-1, and NF-κB transcription factors [30, 32]. Normally, each organ has a higher concentration of specific isotype of the endothelin receptor and accordingly the endothelial of these organs produce the more required endothelin subtype. For instance, the kidney EC, mostly synthesize ET1 and express ETB receptors, to scavenge ET-1 from the plasma and reduce salt and water reabsorption in renal tubules [29]. Endothelin contributes to the extracellular matrix production, and proliferation and migration on vascular EC, vascular smooth muscle cells, fibroblasts, and macrophages/immune cells [33]. Activation of endothelin receptor-B (ET-B) on the EC leads to vasodilation by releasing specific vasodilator mediators and oppositely on the vascular smooth muscle cells where ET-B receptor activation results in vasoconstriction. The ET-A receptor is specific for vascular smooth muscle cells [34]. The biosynthesis of endothelin 1 by the EC is promoted by hormones (epinephrine, cortisol, angiotensin II, vasopressin, insulin), xenobiotics (A23187, cyclosporine), peptides (cytokines; IL-1, TGF-β, endotoxin, ET 1), psychochemical stimuli (hypoxia, shear stress; low), blood components (thrombin, glucose, oxidised LDL) as well as by inhibition of prostacyclin, NO, ANP, BNP, CNP, heparin, shear stress; high [30, 35]. The ET 1 degraded by lysosomal cathepsin A and its inactivation disorders lead to elevation of arterial blood pressure. In the kidney, ET1 induces sodium excretion (diuresis), exerts its effect through ET-B receptors on the epithelial cells of the collecting duct [29]. Several clinical data showed a relationship between ET-1 and the pathogenesis of pulmonary arterial hypertension, vasculopathy, and fibrosis of the skin, lungs, and other organs in systemic sclerosis as well as the potential therapeutic role for endothelin antagonists in these conditions in systemic sclerosis.
Auxiliary vasoconstrictor substances are thromboxane A2, superoxide, their release is usually inflammation-dependent. Both exert their effect in an auto-paracrine manner on their specific EC receptors. However, their effects and receptors are not limited to the ECs, but they can interact directly with VSMCs and induce extra actions (Table 2).
Normally, in the state of inflammation or high blood pressure, the EC shrink, while VSMCs relax to increase interendothelial space and vascular diameter, which is accompanied by hyperpermeability and lower blood pressure. Prostacyclin I2, NO, urotensin, CNP, adrenomedullin, adenosine, purines, reactive oxygen species, and H2S all are vasodilator molecules. NO is a free nitrogen radical released as a byproduct of several cellular activities, including the electron transport chain and under hypoxia and anoxia. Several cells release NO under unfavorable conditions including EC. NO is the most active vasodilator substance and controls the synthesis and activity of vasodilator molecules in the EC. Suggesting, a balance is kept between the endogenous NO production and ET-1/ETA activity to maintain the systematic vascular tone. Endothelial-derived NO release can inhibit the effect of adrenergic innervation on vascular smooth muscle cells [36]. Therefore, the sympathetic tone is predominant in the case of endothelial dysfunction. Currently, it is widely accepted that all adrenergic receptors (β1, 2, 3) contribute to the regulation of vascular tone. However, adrenergic receptors exert their effect on the vessels through the regulation of NO synthase activity, suggesting a NO-dependent vasomotor regulation. Furthermore, the β2 receptor is the predominant vasodilator in large vessels, α2 vasoconstrictor in the peripheral vessels, while β3 was found to enhance eNOS activity in the cardiac chambers and coronary microcirculation, indicating a vasoprotective role [37]. β2 expression is required to sustain a sufficient NO plasma level which is required for maintaining vascular homeostasis, whereas β2 gene silencing in vitro induced oxidative stress and impaired endothelial NO production, confirmed by antioxidant administration superoxide dismutase, reversed the vasoconstriction [37]. The stimulation of the β3 receptor induces angiogenesis. Accumulating evidence, hydrogen peroxide (H2O2) is directly involved in vasorelaxation, whereas, endothelium-dependent relaxation (EDR) involves both pertussis toxin-sensitive Gi include; responses to α2-adrenergic agonists, serotonin, and thrombin, and pertussis toxin-insensitive Gq include; adenosine diphosphate and bradykinin coupling proteins. EDR is promoted by newly discovered stimulators such as insulin, adiponectin [38].
Auxiliary, EC respond to high shear stress by releasing peptide hormone called adrenomedullin, due to activation of the endothelial mechanosensitive cation channel PIEZO1, which induces endothelial NO synthesis by binding of adrenomedullin to its Gs-coupled receptor protein in EC [39]. PIEZO1 activation induces Gq/G11-coupled purinergic P2Y2 receptor activation via an ATP-dependent manner. This required to phosphorylate eNOS at serine 1177/633 which is involved PECAM-1, VE-cadherin, and vascular endothelial growth factor receptor-2 (VEGFR-2)/VEGFR-3 mechanosensitive complex activation [39]. Therefore, adrenomedullin is a marker of hypertension and a guide for the treatment of sepsis and acute heart failure since it shows the severity of endothelial dysfunction including endothelial barrier function disturbance. Adrenomedullin is degraded by neprilysin which is blocked by valsartan, after 22 minutes of lifelong, adrenomedullin is degraded or cleared through binding to its cell surface receptor and internalization then degradation [40]. Finally, NO itself, under certain conditions such as hypoxia, can induce biased activation of soluble guanylyl cyclase promoting the synthesis of cyclic inosine monophosphate instead of cGMP and enhancing contraction rather than relaxation of the beneath VSMCs.
Angiogenesis is a complex process that requires the cooperation of various cell types through signaling exchange between these cell types, aimed at forming new blood vessels from old ones, vascular stem cells, or EC. Angiogenesis or lymphangiogenesis are critical in the progression of many diseases including tumor metastasis and ischemic heart disease [41]. Indeed, EC proliferation-induced-angiogenesis is triggered by several growth factors that are released by the underlying matrix including; FGF-2, TGF-β, TNF-α, IGF-1, and VEGF. However, VEGFR-2 activation, separately able to induce EC proliferation as well as migratory and sprouting activity and to help promote EC to form tubule-like structures. The VEGF combination with chemokines such as angiopoietin-1 and integrins can determine the neovessels’ destination by ephrins migration regulation. The VEGF is required for the initiation of angiogenesis and the formation of immature vessels, while Ang1 and ephrinB2 are responsible for the maturation of initial immature blood vessels. Furthermore, Ang1 expression probably is required for the maintenance of vasculature cells’ identity. On the curbing of EC proliferation is mediated by thrombospondin-1, canstatin, arrestin, tumstatin, angiostatin, and endostatin which are also released by the beneath matrix [6]. Also, the receptor for the vascular endothelial protein tyrosine phosphatase is allocated on the endothelial cell, involved in angiogenesis regulation and membrane fluidity, as well as homeostasis maintaining of the inflamed area through the regulation of the tyrosine kinase receptor Tie-2 and VE-cadherin substrates regulation [42]. Undoubtedly, EC are also involved in vascularization regulation of vascularization.
The endothelial released molecules are also involved in the regulation of angiogenesis such as activated protein c (APC) and vWF [43]. More recently, novel investigations have shown that suppression of vWF expression increases endothelial proliferation, migration, and angiogenesis through binding to integrin αvβ3, and components of Weibel-Palade bodies, such as angiopoietin-2 and galectin-3, whose storage is regulated by vWF, whereas endothelial protein C receptor probably suggests hematopoietic stem cells dormancy and multilineage reconstitution potential [44, 45]. Angiopoietin Ang2 (ANGPT2) binds to tyrosine kinase Tie2 and destabilize blood vessels and synergize with VEGF to promote angiogenesis [46]. Additionally, Gal-3, via the interaction with VEGFR-2 and integrin αvβ3 endothelial cell vWF domains induces angiogenesis, whereas, Gal-3 inhibition, reduces proangiogenesis activity [47]. The Gal-3 regulates cell-cell adhesion which is probably an important step for proangiogenesis [48]. Recent clinical data suggest that an imbalance between Ang1 and Ang2 activity probably underlying the development of the vascular lesions, and maintaining a balance between these growth factors is required for healthy vasculature [49].
Protein c and s are primary anticoagulant peptides synthesized by the liver and platelet as well as EC. Protein c activation requires thrombin-thrombomodulin complex formation on the EC surface, involves proteolysis at Arg169 in EPCR-bound protein c EPCR [50, 51]. Once protein c has been activated, protein s synergistically with calcium and phospholipids cleavage specific arginine polypeptide of proaccelerin and activated antihemophilic factors to inactivate coagulation and promote fibrinolysis by inhibition of tenase and prothrombinase complex [50, 52]. The APC activities are enhanced by protein S, high-density lipoprotein, and glucosylceramide [53]. Furthermore, in animal models, APC induces ERK1/2 (extracellular signal-regulated kinase) during EC proliferation and angiogenesis to promote early growth response factor-1 gene expression through PAR1/S1P1-dependent but EPCR-independent mechanisms solely inhibit TNFα-induced apoptosis in EC [51]. Moreover, in rheumatoid synovial fibroblasts, APC decreases the TNFα-induced phosphorylation of p38 MAPK and JNK [54]. Deficiency of protein s and or c results in DIC syndrome, while activated PC exerts a neuroprotective and neurogenesis effect through G-protein–coupled receptor, protease-activated receptor 1 (PAR1), PAR3, and EPCR that produce signaling at Arg46 site (biased signaling) [53]. The APC binds to EPCR and can cleave PAR-1 and PAR-3, which induce β-arrestin-2–biased PAR1 signaling that results in protection of the vascular endothelium from apoptosis while promoting barrier function. APC reduces the release of neutrophil extracellular trap release [55].
TM is a receptor on the surface receptor of blood and lymphatic vessels thrombin EC, but not only in EC, consisting of 5 domains, each of which has a role in norm and pathology [56]. Thrombomuldin regulates coagulation activity and the release of inflammatory molecules, as well as EC migration via fibronection and ezrin. Besides, its cytoprotective effects interfere with the nuclear translocation of NF-κB and activation of AP-1 [51, 56, 57]. In murine aortic EC, the interaction of the EGF5 domain of TM with G-protein coupled receptor 15 promotes angiogenesis and cell survival through activation of ERK and BCL-2 pathway, suggesting a cytoprotective and angiogenic role of TM on EC [58]. Moreover, endothelial CD39/NTPDase-1 poses an anticoagulant effect by degrading the platelet agonist ADP. TM modulates the duration of pERK nuclear retention and cell proliferation in response to inflammatory stimuli. TM enhances smooth muscle proliferation by activating the ERK pathway through the EGFR axis that negatively affects the growth arrest-specific gene 6, whereas, TM knockdown causes smooth muscle calcification in animal models [59]. Low TM expression by the EC is probably correlated with atherosclerosis development, via the activation of p38 and JNK pathways, where the oxidized low-density lipoprotein reduces the levels of nuclear transcription factors RARβ, RXRα, Sp1, and Sp3 and their binding to TM promoter [51].
When the continuity of endothelium is disrupted or the EC are glycosylated, the coagulation cascade is initiated. EC play a significantly crucial role in maintaining an average level of coagulability, not hyper or hypo clotting. This feature is achieved through the balance between the endothelial released hemostatic factors and the expression of pro and anticoagulant cell surface receptors. In favor of clot formation, EC induce the expression of procoagulant and suppress the expression of anticoagulant factors. Where TNF reduces the formation of TM, endothelial anticoagulant cofactor, and induces procoagulant mechanisms. EC release tissue-type plasminogen activator, plasminogen activator inhibitor type I, platelet activating factor, and vWF into plasma. Exposure to phosphatidylserine induces the release and binding of vWF to collagen fibers and subsequent platelet adhesion to vWF through GP Ib. oxidative stress improves the adhesiveness of vWF, auto association of vWF is regulated primarily by ADAMTS13 protease and is influenced by the level of LDL and HDL, as well as thrombospondin-1 [60, 61]. The arrest by the EC was firstly described in 1882 by Giulio Bizzozero, but later on, appeared that leukocytes roll on the endothelial surface as a secondary phenomenon for coagulation.
The maintenance of transport between the bloodstream and tissues is carried out by EC. Usually, EC perform transcellular (intracellular) or paracellular (intercellular) transport for the fluids, molecules, and leukocytes, paracellular is the dominant form. Intracellular transport requires the formation of caveolae and, through cellular endocytosis, the transported material is internalised, then the intracellular cytoskeleton leads it from the luminal to the abluminal side and in the opposite direction. However, intercellular transport is primarily through specific intercellular tight junctions (e.g., occludins associated with ZO-1, ZO-2, and cingulin proteins) or gap junctions, VE-cadherins (intracellular cytoplasmic proteins and catenins connected to the actin-based microfilament system) [19]. The rearrangement of cadherins and occludins is required to regulate permeability. To note, the VE-cadherins functionality is regulated by small molecules of GTPases that belong to the Rho family, protein phosphorylation, and through interaction with other transmembrane proteins. Several molecules that affect the transport intensity of intercellular transport include; physical forces (e.g., fluid shear stress) and biological mediators (VEGF, FGF, Angiopoietin-1, histamine, sphingosine-1-phosphate) as well as the balance of adhesive forces between EC and between cells and the extracellular matrix and contractile forces generated by the actomyosin system. The loss of barrier function usually arises after disturbance in the regulation mechanisms of the trans or intercellular transport. For instance, under the state of inflammation, the high level of inflammatory mediators in particular histamines induces the intercellular transport of macro and microparticles from the bloodstream to the plasma that results in edema [19].
Endoglin is a TGF-β receptor that positively regulates ALK-1 signaling to Smad1, 5, and 8 but negatively regulates ALK-5-induced Smad2 and Smad3 pathways in EC. ALK-1-mediated activation of Smad1, 5, and 8 EC require the phosphorylation of endoglin in Ser646 and Ser649 by TβRI. The (L)-endoglin comprises a long cytoplasmic tail of 47 amino acid residues, while (S)-endoglin with a short cytoplasmic tail of only 14 amino acid residues. Enhancement of signaling through ALK-1 requires L-endoglin to activate Id1 expression [62]. Whereas, signaling via ALK-5 requires S-endoglin to induce expression of the plasminogen activator inhibitor-1 (PAI-1). The PDZ-binding motif of the cytoplasmic tail of L-endoglin interacts with GAIP interacting protein, carboxyl terminus (GIPC), a scaffolding protein that controls the cell-surface receptor expression and trafficking [62]. When TGF-β-independent interacts with endoglin, GIPC induces phosphorylation of Smad1, 5, and 8 as well as promotes cell-surface retention of endoglin. Furthermore, endoglin binds bone morphogenetic proteins and enhances their signaling via Smad1, 5, and 8. Pathophysiologically, endoglin gene results in deleterious defects in the cardiovascular system, whereas homozygous endoglin gene silencing results in hereditary hemorrhagic telangiectasia type I [62]. Elevation of endoglin is found in preeclampsia [56].
Thromboxane is well known as a vasoconstrictor, through the regulation of endothelial NO synthetase activity. TPr antagonist leads to decrease phosphorylation of eNOS at Ser1177 and Akt at Ser473. Whereas, TPr activation enhances reduction of the Rho-associated kinase- phosphatase and tension homolog deleted on chromosome 10-Akt-eNOS axis, suggesting TPr role in endothelial dysfunction [63]. TPr is involved in the impairment of endothelial function via reduction of NO synthesis and decreased intercellular (EC) gap junction, in turn, this contributes to the development of atherosclerosis and diabetes mellitus as well as hypertension [64].
Not surprising that the EC poses receptors for the angiotensin II, exerts its effect through the Gq receptor signaling cascade, believed that angiotensin regulates the release of vasodilator substances from the EC. Furthermore, angiotensin reduces EC growth and regulates fibrinolysis through the AT2 receptor. Recent findings concluded a direct relationship between angiotensin II receptor type 1 and EC dysfunction through reduction of the endothelial NO synthase phosphorylation via AT 1 R Nox/ROS/PP2A axis, confirmed by AT1 receptor antagonists administration that normalized the NO level via blocking phosphorylation of PP2Ac Tyr307 as well as proved the role of AT-II by knockdown down the p22phox gene, also decreases the phosphorylation of PP2Ac Tyr307 [65].
The EC do pose a cell surface receptor for the serotonin; 1A, 1B, 3A, and 6. A recent investigation suggested an inhibitory role for these receptors, where 1A, 1B, receptors agonist reduced transendothelial cell migration, particularly, allergy-inducing leukocytes [66]. However, EC modify the serotonin-vascular tone activity via regulating the content of serotonin in the vascular bed. Monoamine oxidase type 1 is present in the EC that are directly related to the regulation of the vascular smooth muscle cells.
Recent data were showing that each organ’s EC has its isotype of the muscarinic (M) receptor, however, the M5 receptor approximately is expressed in all EC. The activation of the M3 receptor in healthy endothelium is responsible for vasodilation and oppositely; vasoconstriction in dysfunctional EC [67]. M1 receptors that have been found in cerebral microcirculatory endothelial cells (CMEC) have a relatively low functionality, whereas, highly expressed M3 and M4 receptors [68]. However, the study concluded that all M subtype receptors are expressed by CMECs, particularly in brain vessels and especially M3>M2>M4>M1>M5. This adds more complexity to the surface-expressed receptors of the EC in various organs, probably the same receptors present in the whole vascular tree or organ-specific!
More recently, new data have explained the role of acetylcholine released by the endothelial cell (ACh) in the flow-mediated vasorelaxation, suggesting a stimulatory effect for ACh in calcium channels to release internally stored calcium and induce NO release that leads to vasorelaxation through the previously cAMP pathway [69]. However, ACh production by the EC requires transferring of coenzyme, acetyl‐CoA to choline by a polarized mitochondrial membrane electron transporting complexes, besides, the external supply of choline precursors such as pyruvate was sufficient to induce flow‐evoked ACh production [69].
More recently, new investigations have addressed, scavenger receptor class B type I is an extremely important endothelial receptor, involved in the regulation of HDL transport in the liver, and its dysregulation is believed to be responsible for dyslipidemia and atherosclerosis development [70]. Furthermore, recent investigations were showing that the vitamin D endothelial receptor is directly involved in vasoactivity regulation, particularly in cerebral vessels, where the knockdown of the endothelial vitamin D receptor gene was accompanied by severe vasospasm [71].
Additionally, a small, highly conserved, intracellular calcium-binding protein termed CaM present in the EC. CaM regulates intracellular calcium influx, involved in several physiopathological processes including inflammatory and EC migration inhibition. CaM is required to activate transient receptor potential 6 (TRPC6) channels that later activate the TRPC5 channels to enhance intracellular calcium influx [72]. Also, CaM is involved in the leukocyte paraendothelial migration (TEM) via activating the IQ‐domain GTPase‐activating protein 1 (IQGAP1) and particularly CaM‐binding isoleucine‐glutamine (IQ), inhibition of CaM, theoretically can be used as an anti-inflammatory approach (Table 2) [73].
A bunch of fundamental biological distinctions is present between the lymphatic and blood EC despite the topographical similarities, more than 400 differences in the expressed genes, returned to the difference in the functions of each (Table 3) [78]. The lymphatic EC perform a semi-professional antigen-presenting to the lymphatic circulatory T cytotoxic CD8 cells through invadosome-like protrusion (MHC-I) and express specific surface antigens and receptors such as podoplanin, VEGFR-3, or lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) [79, 80].
Remarkable evidence concluded that sigma receptor-1 of the lymphatic EC is responsible for the regulation of the tone and barrier function as well as the permeability of the lymphatic tree through regulation of nitroxide biosynthesis signaling pathway [20].
Progressive studies in the few previous years established remarkable advances in the molecular biopathology of EC physiopathology. Noticeably, EC present a high degree of heterogeneity in the expression of surface markers (>100 receptors), carrier proteins, and intracellular enzymes, across and within tissues [26]. Hence, EC contribute to maintaining vascular homeostasis as well as regulate organ regeneration capacity after injury [88, 89]. Hypothetically, impaired organs, due to whatever trauma, regenerate only after normalizing blood supply and drainage. Therefore, by applying this concept, EC are directly involved in the recovery of damaged organs. This hypothesis impended the basis for regenerative medicine. Whenever required to enhance the organ’s physiological performance, firstly, it is sufficient to normalize blood circulation, while this required new vessel formation (angiogenesis). Thus, the investigated data emphasizes the endothelial role in the regulation of vital homeostatic metabolic activities.
EC function is influenced by tetrahydrobiopterin (BH4), sex hormones and gender, angiotensin, insulin, vascular endothelial growth factor, physical and psychological stress, vitamin D, adiponectin, uric acid, lipids, oxygen-derived free radicals, aldosterone, and epithelial sodium channels. Probably, perturbance of plasma level of these factors indicates a pre-endothelial dysfunction stage and acts as a clinical marker that usually arises after the failure of the organism to correct/maintain or eliminate the pathological changes in the endothelial influencing factors. The current hypothesis, endothelial function is regulated by the crosstalk between the NO, adrenergic receptors, and oxidative stress. Where, oxidative stress impairs the NO bioavailability via a reduction in the synthesis pathway of the NO and adrenergic effect exerted by NO-dependent manner, while NO plays the central role in the regulation mechanisms. Targeting C-type natriuretic peptide is of great value in inducing angiogenesis [90].
Notes
References
1. Bierhansl L, Conradi LC, Treps L, Dewerchin M, Carmeliet P. 2017; Central role of metabolism in endothelial cell function and vascular disease. Physiology (Bethesda). 32:126–40. DOI: 10.1152/physiol.00031.2016. PMID: 28202623. PMCID: PMC5337830.

2. Khan S, Taverna F, Rohlenova K, Treps L, Geldhof V, de Rooij L, Sokol L, Pircher A, Conradi LC, Kalucka J, Schoonjans L, Eelen G, Dewerchin M, Karakach T, Li X, Goveia J, Carmeliet P. 2019; EndoDB: a database of endothelial cell transcriptomics data. Nucleic Acids Res. 47(D1):D736–44. DOI: 10.1093/nar/gky997. PMID: 30357379. PMCID: PMC6324065.

3. Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, Nishigaki I. 2013; The vascular endothelium and human diseases. Int J Biol Sci. 9:1057–69. DOI: 10.7150/ijbs.7502. PMID: 24250251. PMCID: PMC3831119.

4. Baselet B, Sonveaux P, Baatout S, Aerts A. 2019; Pathological effects of ionizing radiation: endothelial activation and dysfunction. Cell Mol Life Sci. 76:699–728. DOI: 10.1007/s00018-018-2956-z. PMID: 30377700. PMCID: PMC6514067.

5. Zhang J, Tecson KM, McCullough PA. 2021; Role of endothelial cell receptors in the context of SARS-CoV-2 infection (COVID-19). Proc (Bayl Univ Med Cent). 34:262–8. DOI: 10.1080/08998280.2021.1874231. PMID: 33664552. PMCID: PMC7852287.

6. Félétou M. 2011; The endothelium: part 1: multiple functions of the endothelial cells-focus on endothelium-derived vasoactive mediators. Colloq Ser Integr Syst Physiol. 3:1–306. DOI: 10.4199/C00031ED1V01Y201105ISP019. PMID: 21850763.

7. Tian D, Teng X, Jin S, Chen Y, Xue H, Xiao L, Wu Y. 2020; Endogenous hydrogen sulfide improves vascular remodeling through PPARδ/SOCS3 signaling. J Adv Res. 27:115–25. DOI: 10.1016/j.jare.2020.06.005. PMID: 33318871. PMCID: PMC7728593.

8. Shuvaev VV, Brenner JS, Muzykantov VR. 2015; Targeted endothelial nanomedicine for common acute pathological conditions. J Control Release. 219:576–95. DOI: 10.1016/j.jconrel.2015.09.055. PMID: 26435455. PMCID: PMC5450650.

9. Jambusaria A, Hong Z, Zhang L, ivastava S Sr, Jana A, Toth PT, Dai Y, Malik AB, Rehman J. 2020; Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. Elife. 9:e51413. DOI: 10.7554/eLife.51413. PMID: 31944177. PMCID: PMC7002042. PMID: 48432eae5df34dc8902eb012a4ba6d9b.

10. Davies PF. 2009; Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 6:16–26. DOI: 10.1038/ncpcardio1397. PMID: 19029993. PMCID: PMC2851404.

11. Marzoog BA, Vlasova TI. 2022; Apr. 29. The metabolic syndrome puzzles; possible pathogenesis and management. Curr Diabetes Rev. [Epub]. https://doi.org/10.2174/1573399818666220429100411. DOI: 10.2174/1573399818666220429100411. PMID: 35507784.

12. Marzoog BA. 2022; Recent advances in molecular biology of metabolic syndrome pathophysiology: endothelial dysfunction as a potential therapeutic target. J Diabetes Metab Disord. 21:1903–11. DOI: 10.1007/s40200-022-01088-y. PMID: 36065330. PMCID: PMC9430013.

13. Marzoog B. 2022; Lipid behavior in metabolic syndrome pathophysiology. Curr Diabetes Rev. 18:e150921196497. DOI: 10.2174/1573399817666210915101321. PMID: 34525924.

14. Thakore P, Earley S. 2019; Transient receptor potential channels and endothelial cell calcium signaling. Compr Physiol. 9:1249–77. DOI: 10.1002/cphy.c180034. PMID: 31187891. PMCID: PMC6724529.

15. Dalal PJ, Muller WA, Sullivan DP. 2020; Endothelial cell calcium signaling during barrier function and inflammation. Am J Pathol. 190:535–42. DOI: 10.1016/j.ajpath.2019.11.004. PMID: 31866349. PMCID: PMC7074364.

16. Evans CE, Iruela-Arispe ML, Zhao YY. 2021; Mechanisms of endothelial regeneration and vascular repair and their application to regenerative medicine. Am J Pathol. 191:52–65. DOI: 10.1016/j.ajpath.2020.10.001. PMID: 33069720. PMCID: PMC7560161.

17. Hu Y, Chen M, Wang M, Li X. 2022; Flow-mediated vasodilation through mechanosensitive G protein-coupled receptors in endothelial cells. Trends Cardiovasc Med. 32:61–70. DOI: 10.1016/j.tcm.2020.12.010. PMID: 33406458.

18. Pernomian L, do Prado AF, Silva BR, de Paula TD, Grando MD, Bendhack LM. 2021; C-type natriuretic peptide-induced relaxation through cGMP-dependent protein kinase and SERCA activation is impaired in two kidney-one clip rat aorta. Life Sci. 272:119223. DOI: 10.1016/j.lfs.2021.119223. PMID: 33610574.

19. Wettschureck N, Strilic B, Offermanns S. 2019; Passing the vascular barrier: endothelial signaling processes controlling extravasation. Physiol Rev. 99:1467–525. DOI: 10.1152/physrev.00037.2018. PMID: 31140373.

20. Motawe ZY, Abdelmaboud SS, Breslin JW. 2021; Involvement of sigma receptor-1 in lymphatic endothelial barrier integrity and bioenergetic regulation. Lymphat Res Biol. 19:231–9. DOI: 10.1089/lrb.2020.0060. PMID: 33226886. PMCID: PMC8220569.

21. Frees A, Assersen KB, Jensen M, Hansen PBL, Vanhoutte PM, Madsen K, Federlein A, Lund L, Toft A, Jensen BL. 2021; Natriuretic peptides relax human intrarenal arteries through natriuretic peptide receptor type-A recapitulated by soluble guanylyl cyclase agonists. Acta Physiol (Oxf). 231:e13565. DOI: 10.1111/apha.13565. PMID: 33010104.

22. Tao BB, Liu SY, Zhang CC, Fu W, Cai WJ, Wang Y, Shen Q, Wang MJ, Chen Y, Zhang LJ, Zhu YZ, Zhu YC. 2013; VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal. 19:448–64. DOI: 10.1089/ars.2012.4565. PMID: 23199280. PMCID: PMC3704125.
23. Xiao L, Dong JH, Teng X, Jin S, Xue HM, Liu SY, Guo Q, Shen W, Ni XC, Wu YM. 2018; Hydrogen sulfide improves endothelial dysfunction in hypertension by activating peroxisome proliferator-activated receptor delta/endothelial nitric oxide synthase signaling. J Hypertens. 36:651–65. DOI: 10.1097/HJH.0000000000001605. PMID: 29084084.

24. Zuccolo E, Laforenza U, Negri S, Botta L, Berra-Romani R, Faris P, Scarpellino G, Forcaia G, Pellavio G, Sancini G, Moccia F. 2019; Muscarinic M5 receptors trigger acetylcholine-induced Ca2+ signals and nitric oxide release in human brain microvascular endothelial cells. J Cell Physiol. 234:4540–62. DOI: 10.1002/jcp.27234. PMID: 30191989.

25. Berra-Romani R, Faris P, Pellavio G, Orgiu M, Negri S, Forcaia G, Var-Gaz-Guadarrama V, Garcia-Carrasco M, Botta L, Sancini G, Laforenza U, Moccia F. 2020; Histamine induces intracellular Ca2+ oscillations and nitric oxide release in endothelial cells from brain microvascular circulation. J Cell Physiol. 235:1515–30. DOI: 10.1002/jcp.29071. PMID: 31310018.
26. Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. 2018; DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 46(D1):D1074–82. DOI: 10.1093/nar/gkx1037. PMID: 29126136. PMCID: PMC5753335.

27. Tao BB, Cai WJ, Zhu YC. 2015; H2S is a promoter of angiogenesis: identification of H2S "receptors" and its molecular switches in vascular endothelial cells. Handb Exp Pharmacol. 230:137–52. DOI: 10.1007/978-3-319-18144-8_6. PMID: 26162832.

28. Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ. 2016; Endothelin. Pharmacol Rev. 68:357–418. DOI: 10.1124/pr.115.011833. PMID: 26956245. PMCID: PMC4815360.

29. Maguire JJ, Davenport AP. 2015; Endothelin receptors and their antagonists. Semin Nephrol. 35:125–36. DOI: 10.1016/j.semnephrol.2015.02.002. PMID: 25966344. PMCID: PMC4437774.

30. Kowalczyk A, Kleniewska P, Kolodziejczyk M, Skibska B, Goraca A. 2015; The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch Immunol Ther Exp (Warsz). 63:41–52. DOI: 10.1007/s00005-014-0310-1. PMID: 25288367. PMCID: PMC4289534.

31. Abdullah Marzoog B. 2023; Adaptive and compensatory mechanisms of the cardiovascular system and disease risk factors in young males and females. New Emir Med J. 4:e281122211293. DOI: 10.2174/04666221128110145.

32. Marzoog BA. 2022; Oct. 21. Endothelial cell autophagy in the context of disease development. Anat Cell Biol. [Epub]. https://doi.org/10.5115/acb.22.098. DOI: 10.5115/acb.22.098. PMID: 36267005. PMCID: PMC9989784.

33. Motegi S. Takehara K, Fujimoto M, Kuwana M, editors. 2016. Endothelin. Systemic Sclerosis. Springer;p. 155–71. DOI: 10.1007/978-4-431-55708-1_10.

34. Schiffrin EL. 2018; Does endothelin-1 raise or lower blood pressure in humans? Nephron. 139:47–50. DOI: 10.1159/000487346. PMID: 29448245.

35. Houde M, Desbiens L, D'Orléans-Juste P. 2016; Endothelin-1: biosynthesis, signaling and vasoreactivity. Adv Pharmacol. 77:143–75. DOI: 10.1016/bs.apha.2016.05.002. PMID: 27451097.
36. Sousa J, Diniz C. Lenasi H, editor. 2018. Vascular sympathetic neurotransmission and endothelial dysfunction. Endothelial Dysfunction. IntechOpen;DOI: 10.5772/intechopen.72442.

37. Conti V, Russomanno G, Corbi G, Izzo V, Vecchione C, Filippelli A. 2013; Adrenoreceptors and nitric oxide in the cardiovascular system. Front Physiol. 4:321. DOI: 10.3389/fphys.2013.00321. PMID: 24223559. PMCID: PMC3818479. PMID: 736af3961f2b43fe85c5a52e17988f17.

38. Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. 2017; Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiol (Oxf). 219:22–96. DOI: 10.1111/apha.12646. PMID: 26706498.

39. Iring A, Jin YJ, Albarrán-Juárez J, Siragusa M, Wang S, Dancs PT, Nakayama A, Tonack S, Chen M, Künne C, Sokol AM, Günther S, Martínez A, Fleming I, Wettschureck N, Graumann J, Weinstein LS, Offermanns S. 2019; Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J Clin Invest. 129:2775–91. DOI: 10.1172/JCI123825. PMID: 31205027. PMCID: PMC6597232.

40. Voors AA, Kremer D, Geven C, Ter Maaten JM, Struck J, Bergmann A, Pickkers P, Metra M, Mebazaa A, Düngen HD, Butler J. 2019; Adrenomedullin in heart failure: pathophysiology and therapeutic application. Eur J Heart Fail. 21:163–71. DOI: 10.1002/ejhf.1366. PMID: 30592365. PMCID: PMC6607488.

41. Li X, Sun X, Carmeliet P. 2019; Hallmarks of endothelial cell metabolism in health and disease. Cell Metab. 30:414–33. DOI: 10.1016/j.cmet.2019.08.011. PMID: 31484054.

42. Vestweber D. 2021; Vascular endothelial protein tyrosine phosphatase regulates endothelial function. Physiology (Bethesda). 36:84–93. DOI: 10.1152/physiol.00026.2020. PMID: 33595386.

43. Randi AM, Smith KE, Castaman G. 2018; von Willebrand factor regulation of blood vessel formation. Blood. 132:132–40. DOI: 10.1182/blood-2018-01-769018. PMID: 29866817. PMCID: PMC6182264.

44. Nguyen TS, Lapidot T, Ruf W. 2018; Extravascular coagulation in hematopoietic stem and progenitor cell regulation. Blood. 132:123–31. DOI: 10.1182/blood-2017-12-768986. PMID: 29866813. PMCID: PMC6634957.

45. Randi AM, Laffan MA. 2017; Von Willebrand factor and angiogenesis: basic and applied issues. J Thromb Haemost. 15:13–20. DOI: 10.1111/jth.13551. PMID: 27778439.

46. Daly C, Eichten A, Castanaro C, Pasnikowski E, Adler A, Lalani AS, Papadopoulos N, Kyle AH, Minchinton AI, Yancopoulos GD, Thurston G. 2013; Angiopoietin-2 functions as a Tie2 agonist in tumor models, where it limits the effects of VEGF inhibition. Cancer Res. 73:108–18. DOI: 10.1158/0008-5472.CAN-12-2064. PMID: 23149917.

47. Funasaka T, Raz A, Nangia-Makker P. 2014; Galectin-3 in angiogenesis and metastasis. Glycobiology. 24:886–91. DOI: 10.1093/glycob/cwu086. PMID: 25138305. PMCID: PMC4153760.

48. Kim SJ, Chun KH. 2020; Non-classical role of Galectin-3 in cancer progression: translocation to nucleus by carbohydrate-recognition independent manner. BMB Rep. 53:173–80. DOI: 10.5483/BMBRep.2020.53.4.020. PMID: 32172730. PMCID: PMC7196190.

49. Groeneveld DJ, Sanders YV, Adelmeijer J, Mauser-Bunschoten EP, van der Bom JG, Cnossen MH, Fijnvandraat K, Laros-van Gorkom BAP, Meijer K, Lisman T, Eikenboom J, Leebeek FWG. 2018; Circulating angiogenic mediators in patients with moderate and severe von Willebrand disease: a multicentre cross-sectional study. Thromb Haemost. 118:152–60. DOI: 10.1160/TH17-06-0397. PMID: 29304535.

50. Hepner M, Karlaftis V. 2013; Protein C. Methods Mol Biol. 992:365–72. DOI: 10.1007/978-1-62703-339-8_29. PMID: 23546729.

51. Giri H, Cai X, Panicker SR, Biswas I, Rezaie AR. 2019; Thrombomodulin regulation of mitogen-activated protein kinases. Int J Mol Sci. 20:1851. DOI: 10.3390/ijms20081851. PMID: 30991642. PMCID: PMC6514922. PMID: 239b044c6fa24302aea9e478ae983c81.

52. Hepner M, Karlaftis V. 2013; Protein S. Methods Mol Biol. 992:373–81. DOI: 10.1007/978-1-62703-339-8_30. PMID: 23546730.

53. Griffin JH, Zlokovic BV, Mosnier LO. 2018; Activated protein C, protease activated receptor 1, and neuroprotection. Blood. 132:159–69. DOI: 10.1182/blood-2018-02-769026. PMID: 29866816. PMCID: PMC6043978.

54. Julovi SM, Shen K, Mckelvey K, Minhas N, March L, Jackson CJ. 2013; Activated protein C inhibits proliferation and tumor necrosis factor α-stimulated activation of p38, c-Jun NH2-terminal kinase (JNK) and Akt in rheumatoid synovial fibroblasts. Mol Med. 19:324–31. DOI: 10.2119/molmed.2013.00034. PMID: 24096826. PMCID: PMC4344457.

55. Healy LD, Puy C, Fernández JA, Mitrugno A, Keshari RS, Taku NA, Chu TT, Xu X, Gruber A, Lupu F, Griffin JH, McCarty OJT. 2017; Activated protein C inhibits neutrophil extracellular trap formation in vitro and activation in vivo. J Biol Chem. 292:8616–29. DOI: 10.1074/jbc.M116.768309. PMID: 28408624. PMCID: PMC5448091.
56. Loghmani H, Conway EM. 2018; Exploring traditional and nontraditional roles for thrombomodulin. Blood. 132:148–58. DOI: 10.1182/blood-2017-12-768994. PMID: 29866818.

57. Ito T, Kakihana Y, Maruyama I. 2016; Thrombomodulin as an intravascular safeguard against inflammatory and thrombotic diseases. Expert Opin Ther Targets. 20:151–8. DOI: 10.1517/14728222.2016.1086750. PMID: 26558419.

58. Pan B, Wang X, Nishioka C, Honda G, Yokoyama A, Zeng L, Xu K, Ikezoe T. 2017; G-protein coupled receptor 15 mediates angiogenesis and cytoprotective function of thrombomodulin. Sci Rep. 7:692. DOI: 10.1038/s41598-017-00781-w. PMID: 28386128. PMCID: PMC5429650. PMID: cd8c9b4f5e79444090f11c54054dee9b.

59. Son BK, Akishita M, Iijima K, Ogawa S, Arai T, Ishii H, Maemura K, Aburatani H, Eto M, Ouchi Y. 2013; Thrombomodulin, a novel molecule regulating inorganic phosphate-induced vascular smooth muscle cell calcification. J Mol Cell Cardiol. 56:72–80. DOI: 10.1016/j.yjmcc.2012.12.013. PMID: 23274063.

60. Chen J, Chung DW. 2018; Inflammation, von Willebrand factor, and ADAMTS13. Blood. 132:141–7. DOI: 10.1182/blood-2018-02-769000. PMID: 29866815. PMCID: PMC6043979.

61. Marzoog BA, Vlasova TI. 2021; Membrane lipids under norm and pathology. Eur J Clin Exp Med. 19:59–75. DOI: 10.15584/ejcem.2021.1.9.

62. Heldin CH, Moustakas A. 2016; Signaling receptors for TGF-β family members. Cold Spring Harb Perspect Biol. 8:a022053. DOI: 10.1101/cshperspect.a022053. PMID: 27481709. PMCID: PMC4968163.

63. Xie X, Sun W, Wang J, Li X, Liu X, Liu N. 2017; Activation of thromboxane A2 receptors mediates endothelial dysfunction in diabetic mice. Clin Exp Hypertens. 39:312–8. DOI: 10.1080/10641963.2016.1246558. PMID: 28513223.

64. Ellinsworth DC, Shukla N, Fleming I, Jeremy JY. 2014; Interactions between thromboxane A₂, thromboxane/prostaglandin (TP) receptors, and endothelium-derived hyperpolarization. Cardiovasc Res. 102:9–16. DOI: 10.1093/cvr/cvu015. PMID: 24469536.

65. Ding J, Yu M, Jiang J, Luo Y, Zhang Q, Wang S, Yang F, Wang A, Wang L, Zhuang M, Wu S, Zhang Q, Xia Y, Lu D. 2020; Angiotensin II decreases endothelial nitric oxide synthase phosphorylation via AT1R Nox/ROS/PP2A pathway. Front Physiol. 11:566410. DOI: 10.3389/fphys.2020.566410. PMID: 33162896. PMCID: PMC7580705. PMID: d850ebab98074c969807b3972cc481bf.

66. Walker M, Green J, Ferrie R, Cook-Mills J. 2017; Serotonin receptor regulation of eosinophil transendothelial migration. FASEB J. 31:55.
67. Saternos HC, Almarghalani DA, Gibson HM, Meqdad MA, Antypas RB, Lingireddy A, AbouAlaiwi WA. 2018; Distribution and function of the muscarinic receptor subtypes in the cardiovascular system. Physiol Genomics. 50:1–9. DOI: 10.1152/physiolgenomics.00062.2017. PMID: 29093194.
68. Radu BM, Osculati AMM, Suku E, Banciu A, Tsenov G, Merigo F, Di Chio M, Banciu DD, Tognoli C, Kacer P, Giorgetti A, Radu M, Bertini G, Fabene PF. 2017; All muscarinic acetylcholine receptors (M1-M5) are expressed in murine brain microvascular endothelium. Sci Rep. 7:5083. DOI: 10.1038/s41598-017-05384-z. PMID: 28698560. PMCID: PMC5506046. PMID: 02226d4ad5a34ae38427c894c235be2a.
69. Wilson C, Lee MD, McCarron JG. 2016; Acetylcholine released by endothelial cells facilitates flow-mediated dilatation. J Physiol. 594:7267–307. DOI: 10.1113/JP272927. PMID: 27730645. PMCID: PMC5157078.

70. Yu L, Dai Y, Mineo C. 2021; Novel functions of endothelial scavenger receptor class B Type I. Curr Atheroscler Rep. 23:6. DOI: 10.1007/s11883-020-00903-2. PMID: 33420646. PMCID: PMC8918055.
71. Kashefiolasl S, Leisegang MS, Helfinger V, Schürmann C, Pflüger-Müller B, Randriamboavonjy V, Vasconez AE, Carmeliet G, Badenhoop K, Hintereder G, Seifert V, Schröder K, Konczalla J, Brandes RP. 2021; Vitamin D-A new perspective in treatment of cerebral vasospasm. Neurosurgery. 88:674–85. DOI: 10.1093/neuros/nyaa484. PMID: 33269399. PMCID: PMC7884149.

72. Chaudhuri P, Rosenbaum MA, Sinharoy P, Damron DS, Birnbaumer L, Graham LM. 2016; Membrane translocation of TRPC6 channels and endothelial migration are regulated by calmodulin and PI3 kinase activation. Proc Natl Acad Sci U S A. 113:2110–5. DOI: 10.1073/pnas.1600371113. PMID: 26858457. PMCID: PMC4776520.

73. Dalal PJ, Sullivan DP, Muller WA. 2019; Endothelial calmodulin and CaMKII play a role in leukocyte transmigration. FASEB J. 33:375. DOI: 10.1096/fasebj.2019.33.1_supplement.375.5.

74. Shihoya W, Nishizawa T, Okuta A, Tani K, Dohmae N, Fujiyoshi Y, Nureki O, Doi T. 2016; Activation mechanism of endothelin ETB receptor by endothelin-1. Nature. 537:363–8. DOI: 10.1038/nature19319. PMID: 27595334.

75. Xu S, Wen H, Jiang H. 2012; Urotensin II promotes the proliferation of endothelial progenitor cells through p38 and p44/42 MAPK activation. Mol Med Rep. 6:197–200. DOI: 10.3892/mmr.2012.899. PMID: 22552405.
76. Ashraf MA, Nookala V. 2022. Biochemistry of platelet activating factor. StatPearls. StatPearls Publishing;Treasure Island: DOI: 10.1007/springerreference_39453.
77. Ralevic V, Dunn WR. 2015; Purinergic transmission in blood vessels. Auton Neurosci. 191:48–66. DOI: 10.1016/j.autneu.2015.04.007. PMID: 26004513.
78. Berendam SJ, Koeppel AF, Godfrey NR, Rouhani SJ, Woods AN, Rodriguez AB, Peske JD, Cummings KL, Turner SD, Engelhard VH. 2019; Comparative transcriptomic analysis identifies a range of immunologically related functional elaborations of lymph node associated lymphatic and blood endothelial cells. Front Immunol. 10:816. DOI: 10.3389/fimmu.2019.00816. PMID: 31057546. PMCID: PMC6478037. PMID: db1eca5d9c874b308d3564b91c460711.

79. Carman CV, Martinelli R. Bradshaw RA, Stahl PD, editors. 2016. Lymphocyte-endothelial interactions. Encyclopedia of Cell Biology. Elsevier;p. 632–49. DOI: 10.1016/B978-0-12-394447-4.30095-5.

80. Ribatti D. Ribatti D, editor. 2017. The origins of lymphatic vessels: an historical review. Milestones in Immunology. Elsevier;p. 129–62. DOI: 10.1016/B978-0-12-811313-4.00010-3.
81. Keuschnigg J, Karinen S, Auvinen K, Irjala H, Mpindi JP, Kallioniemi O, Hautaniemi S, Jalkanen S, Salmi M. 2013; Plasticity of blood- and lymphatic endothelial cells and marker identification. PLoS One. 8:e74293. DOI: 10.1371/journal.pone.0074293. PMID: 24058540. PMCID: PMC3769239. PMID: aa5f6f4cdead469a84ca43ce114f6dd0.

82. Yang J, Zhang S, Zhang L, Xie X, Wang H, Jie Z, Gu M, Yang JY, Cheng X, Sun SC. 2019; Lymphatic endothelial cells regulate B-cell homing to lymph nodes via a NIK-dependent mechanism. Cell Mol Immunol. 16:165–77. DOI: 10.1038/cmi.2017.167. PMID: 29503445. PMCID: PMC6355805.

83. Russo E, Runge P, Jahromi NH, Naboth H, Landtwing A, Montecchi R, Leicht N, Hunter MC, Takai Y, Halin C. 2021; CD112 regulates angiogenesis and T cell entry into the spleen. Cells. 10:169. DOI: 10.3390/cells10010169. PMID: 33467729. PMCID: PMC7830896. PMID: 48c9bb8f0a614e5d888c34399f17462b.
84. Claro V, Ferro A. 2020; Netrin-1: focus on its role in cardiovascular physiology and atherosclerosis. JRSM Cardiovasc Dis. 9:2048004020959574. DOI: 10.1177/2048004020959574. PMID: 33282228. PMCID: PMC7691900. PMID: 6d1ae565a2334a41a52233a84b211e6e.

85. Dieterich LC, Tacconi C, Menzi F, Proulx ST, Kapaklikaya K, Hamada M, Takahashi S, Detmar M. 2020; Lymphatic MAFB regulates vascular patterning during developmental and pathological lymphangiogenesis. Angiogenesis. 23:411–23. DOI: 10.1007/s10456-020-09721-1. PMID: 32307629. PMCID: PMC7311381.

86. Jourde-Chiche N, Fakhouri F, Dou L, Bellien J, Burtey S, Frimat M, Jarrot PA, Kaplanski G, Le Quintrec M, Pernin V, Rigothier C, Sallée M, Fremeaux-Bacchi V, Guerrot D, Roumenina LT. 2019; Endothelium structure and function in kidney health and disease. Nat Rev Nephrol. 15:87–108. DOI: 10.1038/s41581-018-0098-z. PMID: 30607032.
87. Becker PW, Sacilotto N, Nornes S, Neal A, Thomas MO, Liu K, Preece C, Ratnayaka I, Davies B, Bou-Gharios G, De Val S. 2016; An intronic Flk1 enhancer directs arterial-specific expression via RBPJ-mediated venous repression. Arterioscler Thromb Vasc Biol. 36:1209–19. DOI: 10.1161/ATVBAHA.116.307517. PMID: 27079877. PMCID: PMC4894770.
88. Marzoog BA. 2022; Systemic and local hypothermia in the context of cell regeneration. Cryo Letters. 43:66–73. DOI: 10.54680/fr22210110112. PMID: 36626147.

89. Marzoog BA, Vlasova TI. 2022; Myocardiocyte autophagy in the context of myocardiocytes regeneration: a potential novel therapeutic strategy. Egypt J Med Hum Genet. 23:41. DOI: 10.1186/s43042-022-00250-8. PMID: d1da3ab8dd21477095572d54dc7e6bc3.

90. Bubb KJ, Aubdool AA, Moyes AJ, Lewis S, Drayton JP, Tang O, Mehta V, Zachary IC, Abraham DJ, Tsui J, Hobbs AJ. 2019; Endothelial C-type natriuretic peptide is a critical regulator of angiogenesis and vascular remodeling. Circulation. 139:1612–28. DOI: 10.1161/CIRCULATIONAHA.118.036344. PMID: 30586761. PMCID: PMC6438487.

Fig. 1
EC surface receptors and released molecules. The heterogeneity of the recognized and released molecules by the EC makes them difficult for selective therapeutic targets. In addition, EC regulate and modulate several physiological processes as well as involvement in the pathogenesis of various diseases. EC, endothelial cells; vWF, von Willebrand factor; VEGF, vascular endothelial growth factor.
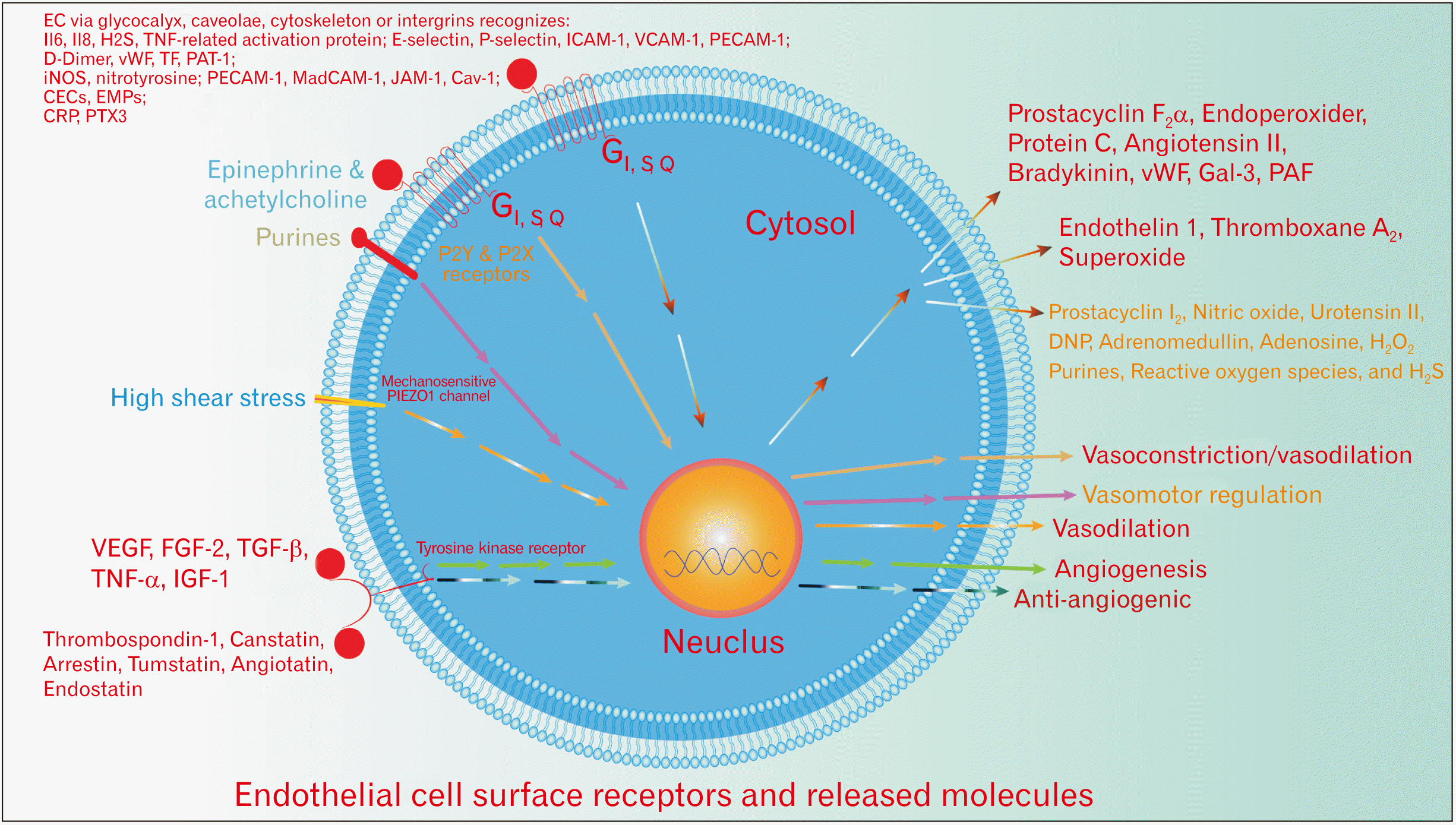
Table 1
Vascular EC surface receptors and their agonist
Table 2
Endothelial released substances and their target receptors as well as their effect
Table 3
Similarities and differences between the VEC and LEC




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download