Abstract
Sjögren syndrome (SS) is a systemic inflammatory autoimmune disease that involves exocrine glands. Shikonin is extracted from comfrey, which is conventionally used as an anti-tumor, antibacterial, and antiviral drug in China. However, the application of Shikonin in SS remains unreported. This study aimed to verify the potential functions of Shikonin in SS progression. Firstly, non-obese diabetic mice were used as the SS mouse model, with C57BL/6 mice serving as the healthy control. It was demonstrated that the salivary gland damage and inflammation were aggravated in the SS mouse model. Shikonin improved salivary gland function decline and injury in the SS mouse model. Moreover, Shikonin reduced inflammatory cytokines and immune infiltration in the SS mouse model. Further experiments discovered that Shikonin attenuated the MAPK signaling pathway in the SS mouse model. Lastly, inhibition of the MAPK signaling pathway combined with Shikonin treatment further alleviated the symptoms of SS. In conclusion, Shikonin ameliorated salivary gland damage and inflammation in a mouse model of SS by modulating the MAPK signaling pathway. Our findings indicate that Shikonin may be a useful drug for SS treatment.
Sjögren syndrome (SS) is a systemic inflammatory autoimmune disease characterized by the destruction and dysfunction of lacrimal and salivary glands, leading to clinical manifestations such as dry mouth and eyes [1]. Infiltrating cells, such as T cells, B cells, epithelial cells, macrophages and dendritic cells, in the salivary glands of SS patients can jointly result in the occurrence of chronic inflammatory reactions [2].
Among causative environmental factors, viral and bacterial infections are closely associated with the incidence of SS. When the glands are subjected with viral infections, toll-like receptors (TLRs) in glandular epithelial cells recognize viral antigens and activate NF-κB and other signaling pathways, increasing cell surface adhesion factors and immune-inflammatory factors [3-5]. After interaction with glandular epithelial cells and dendritic cells, T and B lymphocytes are activated, further stimulating the generation of autoantibodies and inflammatory factors, and enhancing the production of memory B lymphocytes. Consequently, the lymphocytes sequentially act on the target organs, leading to confusion of the immune system [6,7]. Therefore, identifying potential therapeutic drugs that can improve inflammation is a crucial step for treatment of SS.
Shikonin is one of the major useful ingredients of Lithospermum erythrorhizon, a traditional Chinese herb made from the dried roots [8,9]. It has extensive pharmacological effects, including antiviral, anti-tumor, antibacterial, wound healing, and liver protective effects, among others [10-12]. In acetaminophen-mediated acute liver injury, Shikonin treatment can decrease inflammatory factors and suppress oxidative stress, thus alleviating liver injury [13]. Researchers have shown that Shikonin can reduce oxidized-lowdensity lipoprotein (ox-LDL)-stimulated endothelial cell damage through modulating the AMPK/Nrf2/HO-1 signaling pathway, further alleviating atherosclerosis progression [14]. It has been demonstrated that Shikonin has an anti-rheumatic role in the rheumatoid arthritis model by attenuating the PI3K/AKT and MAPK signaling pathways, leading to the inhibition of angiogenesis and the suppression of inflammatory factors [15]. Additionally, Shikonin affects the miR-489-3p/MAP2K1 axis in lipopolysaccharide (LPS)-mediated WI-38 cells to relieve injury and inflammatory response [16]. However, the regulatory functions and mechanisms of Shikonin in SS progression remain unclear.
The MAPK pathway is known to be a vital pathway in the progression of SS [17,18]. It has been reported that the activated MAPK pathway facilitates the proliferation and hyperactivity of T and B lymphocytes, thereby aggravating SS progression [19]. However, whether or not Shikonin regulates the MAPK pathway to affect the progression of SS remains unknown.
In this study, we demonstrated that Shikonin ameliorated salivary gland damage and inflammation in an SS mouse model by modulating the MAPK signaling pathway.
The female C57BL/6 mice (n = 6) and female non-obese diabetic (NOD)/LtJ mice (n = 36) aged 8 weeks were purchased from the Model Animal Research Center of Nanjing University. The C57BL/6 mice (n = 6) group was used as the healthy control. The NOD/LtJ mice (n = 6 for each group) were divided into the NOD, NOD + 12.5 mg/kg Shikonin, NOD + 25 mg/kg Shikonin, NOD + 50 mg/kg Shikonin, NOD + 5 mg/kg SB202190, and NOD + 5 mg/kg SB202190 + 50 mg/kg Shikonin group. Mice were given intraperitoneal injections of Shikonin (12.5, 25, 50 mg/kg) or SB202190 (5 mg/kg) every other day. Salivary flow assays were performed under anesthesia on day 29. All mice were euthanized, and salivary gland tissues were collected. The mice were humanely care for and routinely fed according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health. This study was approved by the Committee of Ethics of Bengbu Medical College (approval number: 2022(325)).
Mice were anesthetized with an intraperitoneal injection of 2.4% pentobarbital sodium (5 ml/kg), followed by an intraperitoneal injection of pilocarpine (5 mg/kg) to stimulate saliva secretion. After 5 min, dry cotton balls were placed in the mouth of the mice to collect saliva, and the cotton balls were weighed again after 10 min. The weight difference of the cotton ball was considered as the saliva flow every 10 min.
After euthanizing the mice, the salivary glands and spleen were collected and weighed. The organ indices were calculated using the following formula: organ index (mg/g) = organ weight (mg)/body weight (g).
The levels of tumor necrosis factor-alpha (TNF-α), interleukin-4 (IL-4), IL-6, IL-17 in serum were evaluated using commercial kits (Abcam) according to the manufacturer’s instructions.
Total proteins were extracted from salivary glands tissues using RIPA lysis buffer. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (10%) was used for protein electrophoresis. The proteins were then transferred to the polyvinylidene difluoride (PVDF) membranes. After blocking with 5% bovine serum albumin, the membranes were incubated with diluted primary antibodies for 12 h at 4°C. After washing, the PDVF membranes were incubated with diluted goat anti-rabbit IgG H&L (HRP) (1:5,000, ab6721; Abcam), goat anti-mouse IgG H&L (HRP) (1:5,000, ab205719; Abcam) at room temperature for 2 h. The bands were detected using the ECL system (Bio-Rad) and analyzed using Image J. The primary antibodies used were p-NF-κB (1:1,000, ab239882; Abcam), NF-κB (1:1,000, ab207297), p-ERK (1:1,000, ab201015), ERK (1:10,000, ab184699), p-JNK (1:5,000, ab76572), JNK (1:1,000, ab179461), p-P38 (1:1,000, ab45381), P38 (1:1,000, ab170099), and β-actin (1 µg/ml; ab8226).
The salivary glands of mice were homogenized and filtered through a 200 µm-mesh filter to obtain a single cell suspension. The cells were resuspended in 1 ml of RPMI-1640 medium at a concentration of 4 × 106 cells/ml, and incubated with a cell stimulant (10 µl) at 37°C for 4 h. After washing, the cells were resuspended and incubated with CD4 (GK1.5), CD8 (2.43), CD19 (1D3), and B220 (RA3-6B2) for 15 min in the dark at room temperature. After washing, positive cells (1 × 104) were collected from each group and analyzed by flow cytometry (BD Biosciences). The percentage of positive cells was analyzed using the FLOWJO software.
Spleen and thymus tissues were filtered through a sterile 200 µm-mesh filter to obtain spleen B lymphocytes and thymus T lymphocytes in PBS. The lymphocyte suspension (50 μl) was placed into a 96-well plate with RPMI-1640 (10% FBS). LPS (50 μl) and Concanavalin A (50 μl) were used to stimulate B and T lymphocytes, respectively. After 48 h of incubation, each well was mixed with 10 μl CCK-8 solution (Beyotime), and the absorbance at 450 nm was assessed using a spectrophotometer (Thermo Fisher Scientific).
Statistical analyses were performed using GraphPad Prism software, version 9.0 (GraphPad Software). Data are presented as mean ± standard deviation. The differences were compared using Student’s t-test (for two groups) and one-way analysis of variance for multiple groups. A p-value of < 0.05 was considered statistically significant.
The salivary flow was found to reduce in NOD group, but this effect was offset after treatment with Shikonin (Fig. 1A). Moreover, the effects of Shikonin were enhanced with increasing doses (12.5, 25, and 50 mg/kg). The salivary gland index and spleen index were increased in NOD mice (p < 0.001), but these changes were weakened after Shikonin treatment (p < 0.001) (Fig. 1B). The 50 mg/kg dose of Shikonin exhibited the strongest effect, with a decrease of approximately 50%. Taken together, Shikonin improved salivary gland function decline and injury in the SS mouse model.
Inflammation is a crucial progress in SS, and thus the effects of Shikonin on inflammation were investigated. ELISA results demonstrated that the levels of TNF-α, IL-6, IL-17 and IL-4 in the blood were all increased in the SS mouse model (p < 0.001), but these effects were reversed after treatment with Shikonin (Fig. 2A). Furthermore, the level of p-NF-κB/NF-κB in the blood was elevated in the SS mouse model (p < 0.001), but this effect was attenuated after Shikonin treatment (Fig. 2B). Similarly, the 50 mg/kg dose of Shikonin exhibited the strongest effect, with a decrease of more than 50%. These data suggest that Shikonin reduced inflammatory cytokines in the SS mouse model.
Immune responses are closely associated with SS progression, including the CD4+, CD8+, B220+CD19+ labeled T and B lymphocytes; therefore, these cells were further explored. The results demonstrated that the percentages of CD4+, CD8+ and B220+CD19+ were heightened in the SS mouse model (p < 0.001), but these changes were reduced after Shikonin treatment (Fig. 3A). In addition, the cell viabilities of B and T cells were both increased in the SS mouse model (p < 0.001), but these effects were reversed after Shikonin treatment (Fig. 3B, C). Among them, Shikonin at 50 mg/kg had the best remission effect with about a 50% decrease. These findings indicates that Shikonin reduced immune infiltration in the SS mouse model.
The MAPK signaling pathway is activated in SS progression; therefore, this study explored whether Shikonin could improve SS symptoms by regulating the MAPK signaling pathway. Further experiments demonstrated that the levels of p-ERK/ERK, p-JNK/JNK and p-P38/P38 were up-regulated in the SS mouse model (p < 0.001), but these effects were reduced after Shikonin treatment (Fig. 4). Overall, Shikonin attenuated the MAPK signaling pathway in the SS mouse model.
Lastly, the MAPK pathway inhibitor SB202190 was used. The results revealed that the decreased salivary flow in the SS mouse model was reversed after SB202190 treatment (p < 0.001), and this remission effect was further strengthened by the addition of Shikonin (p < 0.001) (Fig. 5A). The enhanced salivary gland index and spleen index in the NOD group were relieved by SB202190 treatments (p < 0.001), and the amelioration effect was facilitated by the combined treatments of SB202190 and Shikonin (Fig. 5B). Additionally, the elevated TNF-α, IL-6, IL-17, IL-4 levels in the NOD group were reduced after SB202190 treatment (p < 0.001), and this attenuation effect was further strengthened by the addition of Shikonin (Fig. 5C). The increased percentages of CD4+, CD8+, B220+CD19+ in the NOD group were attenuated after SB202190 treatment (p < 0.001), and this remission change was further heightened by the addition of Shikonin (Fig. 5D). In summary, the inhibition of the MAPK signaling pathway, combined with Shikonin treatment, alleviates symptoms of SS.
Various drugs have been investigated to improve SS progression. For example, Catalpol regulates the interplay between B and T cells, leading to an improvement in SS progression [20]. Additionally, Acteoside accelerates B cell-derived IL-10 production and relieves autoimmunity in SS [21]. Furthermore, Triptolide modulates the JAK/STAT and NF-κB signaling pathways in a NOD mouse model of SS to improve salivary gland damage [22]. Currently, the role of Shikonin in SS progression is unclear, although it has been shown to have regulatory functions in some diseases [13-16]. Shikonin has also been found to have systemic toxicity against regular cell lines [23]. The toxicity of large doses of Shikonin may affect SS progression; therefore, the three different doses (12.5, 25, and 50 mg/kg) were chosen based on previous studies [24,25].
In this study, NOD/LtJ mice were used as the SS mouse model, with B57BL/6 mice serving as the healthy control. The study demonstrated that salivary gland damage and inflammation were aggravated in the SS mouse model. However, Shikonin improved salivary gland function decline and injury in the SS mouse model. Moreover, Shikonin reduced inflammatory cytokines and immune infiltration in the SS mouse model.
The MAPK pathway has been identified as a key pathway that affects inflammation in some diseases. For example, Indirubin modulates the TLR4 abrogation-mediated NF-κB and MAPK pathways, leading to a reduction in LPS-stimulated inflammation [26]. Additionally, the p38-MAPK signaling pathway affects the NLRP3 inflammasome and macrophage pyroptosis, regulating acute lung injury [27]. LL-37 regulates the MAPK signaling pathway to suppress LPS-mediated inflammation and induce the osteogenic differentiation of bone marrow mesenchymal stem cells [28]. Furthermore, circPrkcsh modulates the JNK/p38 MAPK pathway to facilitate the microglia M1 polarization in spinal cord injury [29].
The MAPK pathway also has been investigated in the regulation of SS progression. For instance, in the NOD/Ltj mouse model, suppression of the TLR9-dependent p38-MAPK pathway can ameliorate primary SS progression [30]. LncRNA Neat1 participates in SS progression by positively regulating the MAPK pathway [31]. Additionally, IL-17 actives the p38-MAPK, ERK, and NF-κB pathways in SS, enhancing t IL-6 expression [32]. Moreover, TLR9 signaling affects the p38/JNK MAPK pathway in SS, aggravating autophagy and apoptosis [33]. However, it remains unknown whether Shikonin regulates the MAPK pathway to affect SS progression. In this study, further experiments revealed that Shikonin attenuated the MAPK signaling pathway in the SS mouse model. Lastly, inhibition of the MAPK signaling pathway (using SB202190) combined with Shikonin treatment further alleviated the symptoms of SS.
In conclusion, Shikonin was found to ameliorate salivary gland damage and inflammation in the SS mouse model by modulating the MAPK signaling pathway. Our findings suggest that Shikonin may be a promising drug for SS treatment. However, this study has some limitations in terms of its exploration of the effects of Shikonin on SS progression, such as the lack of human samples and investigation of other phenotypes. Future experiments should be conducted to further explore the regulatory functions of Shikonin in SS progression.
REFERENCES
1. Nakano Y, Yamamoto M, Komatsu K, Yagita M, Fujita M. 2018; Hypertrophic pachymeningitis in Sjögren's syndrome. Intern Med. 57:413–415. DOI: 10.2169/internalmedicine.9406-17. PMID: 29093421. PMCID: PMC5827326.

2. Sumida T, Azuma N, Moriyama M, Takahashi H, Asashima H, Honda F, Abe S, Ono Y, Hirota T, Hirata S, Tanaka Y, Shimizu T, Nakamura H, Kawakami A, Sano H, Ogawa Y, Tsubota K, Ryo K, Saito I, Tanaka A, et al. 2018; Clinical practice guideline for Sjögren's syndrome 2017. Mod Rheumatol. 28:383–408. DOI: 10.1080/14397595.2018.1438093. PMID: 29409370.

3. Imgenberg-Kreuz J, Sandling JK, Nordmark G. 2018; Epigenetic alterations in primary Sjögren's syndrome - an overview. Clin Immunol. 196:12–20. DOI: 10.1016/j.clim.2018.04.004. PMID: 29649576.

4. Verstappen GM, Meiners PM, Corneth OBJ, Visser A, Arends S, Abdulahad WH, Hendriks RW, Vissink A, Kroese FGM, Bootsma H. 2017; Attenuation of follicular helper T cell-dependent B cell hyperactivity by abatacept treatment in primary Sjögren's syndrome. Arthritis Rheumatol. 69:1850–1861. DOI: 10.1002/art.40165. PMID: 28564491.

5. Nakamura H, Hasegawa H, Sasaki D, Takatani A, Shimizu T, Kurushima S, Horai Y, Nakashima Y, Nakamura T, Fukuoka J, Kawakami A. 2018; Detection of human T lymphotropic virus type-I bZIP factor and tax in the salivary glands of Sjögren's syndrome patients. Clin Exp Rheumatol. 36 Suppl 112:51–60.
6. Ranta N, Valli A, Grönholm A, Silvennoinen O, Isomäki P, Pesu M, Pertovaara M. 2018; Proprotein convertase enzyme FURIN is upregulated in primary Sjögren's syndrome. Clin Exp Rheumatol. 36 Suppl 112:47–50.
7. Song C, Adili A, Kari A, Abuduhaer A. 2021; FSTL1 aggravates sepsis-induced acute kidney injury through regulating TLR4/MyD88/NF-κB pathway in newborn rats. Signa Vitae. 17:167–173. DOI: 10.22514/sv.2021.071.
8. Matsui K, Sano H. 2017; T helper 17 cells in primary Sjögren's syndrome. J Clin Med. 6:65. DOI: 10.3390/jcm6070065. PMID: 28678161. PMCID: PMC5532573. PMID: 9fcdfb9c61ce465aa221b7dbe1bc37ba.

9. Nicaise C, Weichselbaum L, Schandene L, Gangji V, Dehavay F, Bouchat J, Balau B, Vogl T, Soyfoo MS. 2017; Phagocyte-specific S100A8/A9 is upregulated in primary Sjögren's syndrome and triggers the secretion of pro-inflammatory cytokines in vitro. Clin Exp Rheumatol. 35:129–136. PMID: 27749214.
10. Sun Q, Gong T, Liu M, Ren S, Yang H, Zeng S, Zhao H, Chen L, Ming T, Meng X, Xu H. 2022; Shikonin, a naphthalene ingredient: therapeutic actions, pharmacokinetics, toxicology, clinical trials and pharmaceutical researches. Phytomedicine. 94:153805. DOI: 10.1016/j.phymed.2021.153805. PMID: 34749177.
11. Yadav S, Sharma A, Nayik GA, Cooper R, Bhardwaj G, Sohal HS, Mutreja V, Kaur R, Areche FO, AlOudat M, Shaikh AM, Kovács B, Mohamed Ahmed AE. 2022; Review of shikonin and derivatives: isolation, chemistry, biosynthesis, pharmacology and toxicology. Front Pharmacol. 13:905755. DOI: 10.3389/fphar.2022.905755. PMID: 35847041. PMCID: PMC9283906. PMID: 60cd9eec9c384004aaf944a7253df391.

12. Cao HH, Liu DY, Lai YC, Chen YY, Yu LZ, Shao M, Liu JS. 2020; Inhibition of the STAT3 signaling pathway contributes to the anti-melanoma activities of shikonin. Front Pharmacol. 11:748. DOI: 10.3389/fphar.2020.00748. PMID: 32536866. PMCID: PMC7267064. PMID: 11b0e8a5b01243ac8a37752c12988778.
13. Huang XY, Fu HL, Tang HQ, Yin ZQ, Zhang W, Shu G, Yin LZ, Zhao L, Yan XR, Lin JC. 2020; Optimization extraction of shikonin using ultrasound-assisted response surface methodology and antibacterial studies. Evid Based Complement Alternat Med. 2020:1208617. DOI: 10.1155/2020/1208617. PMID: 32802111. PMCID: PMC7411493.

14. Figat R, Zgadzaj A, Geschke S, Sieczka P, Pietrosiuk A, Sommer S, Skrzypczak A. 2021; Cytotoxicity and antigenotoxicity evaluation of acetylshikonin and shikonin. Drug Chem Toxicol. 44:140–147. DOI: 10.1080/01480545.2018.1536710. PMID: 30574814.

15. Guo H, Sun J, Li D, Hu Y, Yu X, Hua H, Jing X, Chen F, Jia Z, Xu J. 2019; Shikonin attenuates acetaminophen-induced acute liver injury via inhibition of oxidative stress and inflammation. Biomed Pharmacother. 112:108704. DOI: 10.1016/j.biopha.2019.108704. PMID: 30818140.

16. Liu S, Yan W, Hu Y, Wu H. 2021; Shikonin alleviates endothelial cell injury induced by ox-LDL via AMPK/Nrf2/HO-1 signaling pathway. Evid Based Complement Alternat Med. 2021:5881321. DOI: 10.1155/2021/5881321. PMID: 34912465. PMCID: PMC8668324.

17. Liu C, He L, Wang J, Wang Q, Sun C, Li Y, Jia K, Wang J, Xu T, Ming R, Wang Q, Lin N. 2020; Anti-angiogenic effect of Shikonin in rheumatoid arthritis by downregulating PI3K/AKT and MAPKs signaling pathways. J Ethnopharmacol. 260:113039. DOI: 10.1016/j.jep.2020.113039. PMID: 32497675.

18. Wang J, Chen Z, Feng X, Yin L. 2021; Shikonin ameliorates injury and inflammatory response of LPS-stimulated WI-38 cells via modulating the miR-489-3p/MAP2K1 axis. Environ Toxicol. 36:1775–1784. DOI: 10.1002/tox.23298. PMID: 34089293.

19. Ma X, Zou J, He L, Zhang Y. 2014; Dry eye management in a Sjögren's syndrome mouse model by inhibition of p38-MAPK pathway. Diagn Pathol. 9:5. DOI: 10.1186/1746-1596-9-5. PMID: 24443980. PMCID: PMC3976089.

20. Ren Y, Cui G, Gao Y. 2021; Research progress on inflammatory mechanism of primary Sjögren syndrome. Zhejiang Da Xue Xue Bao Yi Xue Ban. 50:783–794. DOI: 10.3724/zdxbyxb-2021-0072. PMID: 35347914. PMCID: PMC8931614.
21. Chen X, Zhang P, Liu Q, Zhang Q, Gu F, Xu S, Körner H, Wu H, Wei W. 2020; Alleviating effect of paeoniflorin-6'-O-benzene sulfonate in antigen-induced experimental Sjögren's syndrome by modulating B lymphocyte migration via CXCR5-GRK2-ERK/p38 signaling pathway. Int Immunopharmacol. 80:106199. DOI: 10.1016/j.intimp.2020.106199. PMID: 31955068.

22. Lin R, Hao D, Dong Y, Wang Y. 2020; Catalpol ameliorates Sjögren's syndrome by modulating interplay of T and B cells. Biomed Pharmacother. 123:109806. DOI: 10.1016/j.biopha.2019.109806. PMID: 31951976.

23. Wu M, Yu S, Chen Y, Meng W, Chen H, He J, Shen J, Lin X. 2022; Acteoside promotes B cell-derived IL-10 production and ameliorates autoimmunity. J Leukoc Biol. 112:875–885. DOI: 10.1002/JLB.3MA0422-510R. PMID: 35638582.

24. Guo Y, Ji W, Lu Y, Wang Y. 2021; Triptolide reduces salivary gland damage in a non-obese diabetic mice model of Sjögren's syndrome via JAK/STAT and NF-κB signaling pathways. J Clin Biochem Nutr. 68:131–138. DOI: 10.3164/jcbn.20-15. PMID: 33879964. PMCID: PMC8046007.

25. Wiench B, Eichhorn T, Paulsen M, Efferth T. 2012; Shikonin directly targets mitochondria and causes mitochondrial dysfunction in cancer cells. Evid Based Complement Alternat Med. 2012:726025. DOI: 10.1155/2012/726025. PMID: 23118796. PMCID: PMC3478753.

26. Bai GZ, Yu HT, Ni YF, Li XF, Zhang ZP, Su K, Lei J, Liu BY, Ke CK, Zhong DX, Wang YJ, Zhao JB. 2013; Shikonin attenuates lipopolysaccharide-induced acute lung injury in mice. J Surg Res. 182:303–311. DOI: 10.1016/j.jss.2012.10.039. PMID: 23158409.

27. Wang Z, Liu T, Gan L, Wang T, Yuan X, Zhang B, Chen H, Zheng Q. 2010; Shikonin protects mouse brain against cerebral ischemia/reperfusion injury through its antioxidant activity. Eur J Pharmacol. 643:211–217. DOI: 10.1016/j.ejphar.2010.06.027. PMID: 20599918.

28. Lai JL, Liu YH, Liu C, Qi MP, Liu RN, Zhu XF, Zhou QG, Chen YY, Guo AZ, Hu CM. 2017; Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways. Inflammation. 40:1–12. DOI: 10.1007/s10753-016-0447-7. PMID: 27718095.

29. Li D, Ren W, Jiang Z, Zhu L. 2018; Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Mol Med Rep. 18:4399–4409. DOI: 10.3892/mmr.2018.9427.

30. Yu X, Quan J, Long W, Chen H, Wang R, Guo J, Lin X, Mai S. 2018; LL-37 inhibits LPS-induced inflammation and stimulates the osteogenic differentiation of BMSCs via P2X7 receptor and MAPK signaling pathway. Exp Cell Res. 372:178–187. DOI: 10.1016/j.yexcr.2018.09.024. PMID: 30287143.

31. Li X, Kang J, Lv H, Liu R, Chen J, Zhang Y, Zhang Y, Yu G, Zhang X, Ning B. 2021; CircPrkcsh, a circular RNA, contributes to the polarization of microglia towards the M1 phenotype induced by spinal cord injury and acts via the JNK/p38 MAPK pathway. FASEB J. 35:e22014. DOI: 10.1096/fj.202100993R.

32. Cao N, Shi H, Chen C, Zheng L, Yu C. 2021; Inhibition of the TLR9-dependent p38 MAPK signaling pathway improves the pathogenesis of primary Sjögren's syndrome in the NOD/Ltj mouse. J Biol Regul Homeost Agents. 35:1103–1108. DOI: 10.23812/21-154-L. PMID: 34034463.
33. Ye L, Shi H, Yu C, Fu J, Chen C, Wu S, Zhan T, Wang B, Zheng L. 2020; LncRNA Neat1 positively regulates MAPK signaling and is involved in the pathogenesis of Sjögren's syndrome. Int Immunopharmacol. 88:106992. DOI: 10.1016/j.intimp.2020.106992. PMID: 33182021.

34. Wei L, Xiong H, Li W, Li B, Cheng Y. 2018; Upregulation of IL-6 expression in human salivary gland cell line by IL-17 via activation of p38 MAPK, ERK, PI3K/Akt, and NF-κB pathways. J Oral Pathol Med. 47:847–855. DOI: 10.1111/jop.12765. PMID: 30007092.

35. Fu J, Shi H, Cao N, Wu S, Zhan T, Xie L, Wang Z, Ye L, Li C, Shen Y, Yu C, Zheng L. 2019; Toll-like receptor 9 signaling promotes autophagy and apoptosis via divergent functions of the p38/JNK pathway in human salivary gland cells. Exp Cell Res. 375:51–59. DOI: 10.1016/j.yexcr.2018.12.027. PMID: 30610847.

Fig. 1
Shikonin improves salivary gland function decline and injury in the SS mouse model.
Groups were divided into five groups: C57BL/6, NOD, NOD + 12.5 mg/kg Shikonin, NOD + 25 mg/kg Shikonin, and NOD + 50 mg/kg Shikonin (n = 6 for each group). (A) Salivary flow (mg/10 min) was measured. ***p < 0.001 vs. the C57BL/6 group, ###p < 0.001 vs. the NOD group. (B) Salivary gland index (mg/g) and spleen index (mg/g) were determined. ***p < 0.001. Values are presented as mean ± SD. SS, Sjögren syndrome; NOD, non-obese diabetic; ns, not significant.
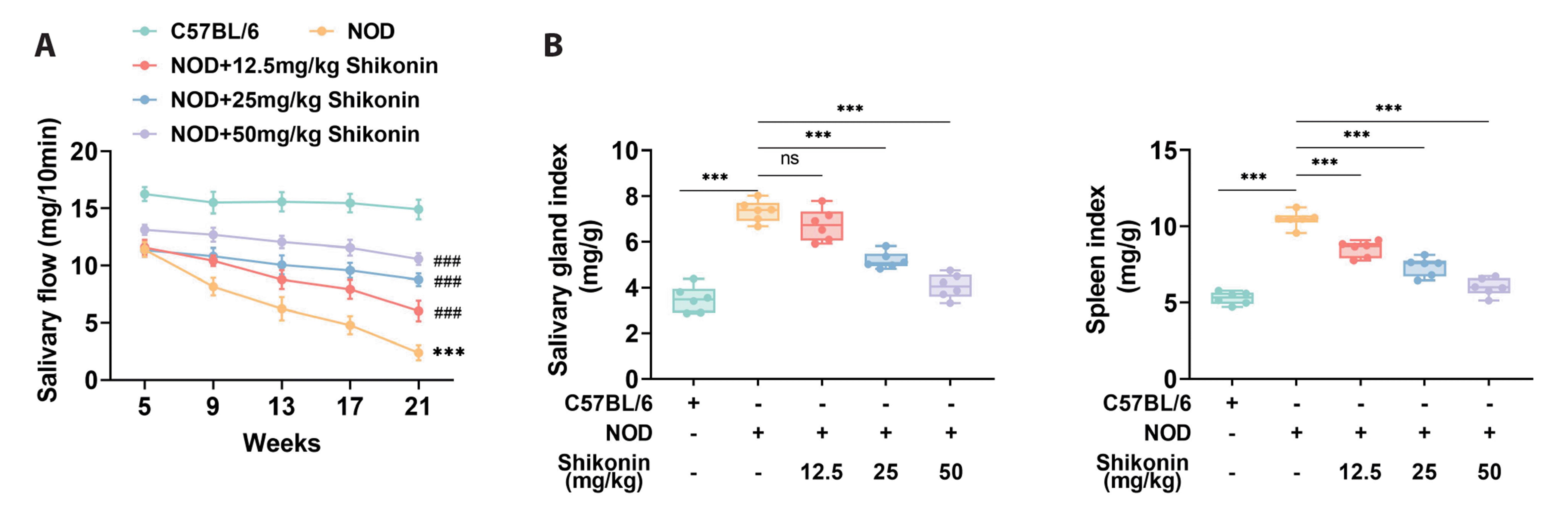
Fig. 2
Shikonin reduces inflammatory cytokines in the SS mouse model.
Groups were divided into five groups: C57BL/6, NOD, NOD + 12.5 mg/kg Shikonin, NOD + 25 mg/kg Shikonin, and NOD + 50 mg/kg Shikonin. (A) The levels of TNF-α, IL-6, IL-17, IL-4 in the blood were measured using ELISA. (B) The protein expressions of p-NF-κB, NF-κB in the blood were analyzed using Western blot. *p < 0.05, ***p < 0.001. Values are presented as mean ± SD. SS, Sjögren syndrome; NOD, non-obese diabetic; ns, not significant.
Fig. 3
Shikonin reduces immune infiltration in the SS mouse model.
Groups were divided into five groups: C57BL/6, NOD, NOD + 12.5 mg/kg Shikonin, NOD + 25 mg/kg Shikonin, and NOD + 50 mg/kg Shikonin. (A) Flow cytometry was used to detect the percentages of CD4+, CD8+, and B220+CD19+ cells. (B, C) The cell viability of B and T cells was measured using the CCK-8 assay. *p < 0.05, **p < 0.01, ***p < 0.001. Values are presented as mean ± SD. SS, Sjögren syndrome; NOD, non-obese diabetic; ns, not significant.
Fig. 4
Shikonin modulates the MAPK signaling pathway.
Groups were divided into five groups: C57BL/6, NOD, NOD + 12.5 mg/kg Shikonin, NOD + 25 mg/kg Shikonin, and NOD + 50 mg/kg Shikonin. Western blot was used to examine the protein expressions of p-ERK, ERK, p-JNK, JNK, p-P38, and P38. ***p < 0.001. Values are presented as mean ± SD. NOD, non-obese diabetic.
Fig. 5
Inhibition of the MAPK signaling pathway alleviates the symptoms of SS.
Groups were divided into four groups: C57BL/6, NOD, NOD + 5 mg/kg SB202190, NOD + 5 mg/kg SB202190 + 50 mg/kg Shikonin. (A) Salivary flow (mg/10 min) was examined. ***p < 0.001 vs. the C57BL/6 group, ###p < 0.001 vs. the NOD group, ^^^p < 0.001 vs. the NOD + 5 mg/kg SB202190 group. (B) The salivary gland index (mg/g) and spleen index (mg/g) were measured. (C) ELISA was used to verify the levels of TNF-α, IL-6, IL-17, and IL-4. (D) Flow cytometry was used to detect the percentages of CD4+, CD8+, and B220+CD19+ cells. *p < 0.05, **p < 0.01, ***p < 0.001. Values are presented as mean ± SD. SS, Sjögren syndrome; NOD, non-obese diabetic.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download