Abstract
This paper reports a 70-year-old female with gastric extranodal marginal zone B cell lymphoma of mucosa-associated lymphoid tissue (gastric MALT lymphoma) as a rare case of gastric outlet obstruction. Five years earlier, she initially presented with weight loss and anemia. Esophagogastroduodenoscopy (EGD) revealed multiple gastric and duodenal ulcers with a pyloric deformity, while histology revealed chronic active inflammation and a Helicobacter pylori (H. pylori) infection. Three years earlier, she underwent EGD per the National Cancer Screening Program and was diagnosed with antral and duodenal ulcers. A forceps biopsy specimen from one of the ulcers showed the findings of gastric MALT lymphoma, but she did not visit the hospital for proper management. She visited complaining of a loss of appetite. EGD revealed a gastric outlet obstruction (GOO) caused by antral deformity and pyloric narrowing. A staged workup with CT and PET revealed full-layered, encircling antral wall thickening and several enlarged mesenteric lymph nodes. She was finally diagnosed with a gastric MALT lymphoma at Ann Arbor stage I1E with translocation t(11;18). She was treated with palliative surgery for GOO and systemic chemotherapy with a CHOP regimen. This paper reports a gastric MALT lymphoma that progressed from superficial mucosal lesions to an overt mass with regional lymph node metastasis for five years.
An extranodal marginal zone B-cell lymphoma of the mucosa- associated lymphoid tissue (MALT), also known as a MALT lymphoma, is a type of lymphoma characterized as the malignant transformation of B cells from the marginal zone of MALT.1 The stomach is the most prevalent site of a MALT lymphoma. A gastric MALT lymphoma typically follows an indolent clinical course and is a localized disease.2
A Helicobacter pylori (H. pylori) infection is closely associated with the pathogenesis of gastric MALT lymphoma,3 and H. pylori eradication can induce the regression of tumorigenesis of gastric MALT lymphoma even in an advanced stage. More than 75% of cases of gastric MALT lymphoma achieve remission with H. pylori eradication,4 and H. pylori eradication is recommended for advanced and localized disease, regardless of the need for additional treatment.
Owing to the indolent nature of the gastric MALT lymphoma, complications, such as obstruction, perforation, or bleeding, are rarely encountered, particularly in Korea, where the two-year interval esophagogastroduodenoscopy (EGD) is provided for every individual age 40 or over. This paper reports a case of gastric MALT lymphoma presenting as a gastric outlet obstruction following five years of an untreated clinical course.
Five years earlier, a 70-year-old female visited a gastroenterology clinic presenting with anemia and unintentional weight loss of 5.0 kg for the previous eight months. She had been treated for hypertension for five years and had a history of a brain infarction. Her family history was insignificant. The laboratory values were notable for decreased hemoglobin (8.8 g/dL) with low iron and a lower normal range of ferritin and total iron binding capacity, indicating iron-deficiency anemia and an anemia of chronic disease pattern. Esophagogastroduodenoscopy (EGD) showed multiple gastric and duodenal ulcers with pyloric deformities (Fig. 1A). A histological examination revealed chronic active inflammation with lymphoid follicles and aggregations and a H. pylori infection. She was prescribed lansoprazole 30 mg daily for eight weeks to treat the peptic ulcers, and H. pylori eradication was planned to begin after ulcer healing, but she did not complete a follow-up visit.
Two years later, she underwent EGD as a National Cancer Screening Program participant and was diagnosed with multiple peptic ulcers. The ulcers were scattered in the stomach and duodenum and showed various stages (Fig. 1B). The histological examination revealed a H. pylori infection on Warthin-Starry silver staining and atypical lymphoid hyperplasia on the background of chronic active gastritis. The H. pylori eradication regimen was prescribed as 10-day sequential therapy (rabeprazole 20 mg bid daily plus amoxicillin 1.0 g bid daily for the first five days followed by rabeprazole 20 mg bid daily plus clarithromycin 500 mg bid daily plus metronidazole 500 mg tid daily for the following five days). Immunohistochemical staining for cytokeratin AE1/AE3, CD3, CD5, and CD20 was performed, and a follow-up visit and EGD were scheduled to confirm the histological suspicion of gastric MALT lymphoma. A further histological evaluation revealed B cell monoclonality with the lymphoepithelial lesion, but she did not attend a follow-up visit again for the following three years.
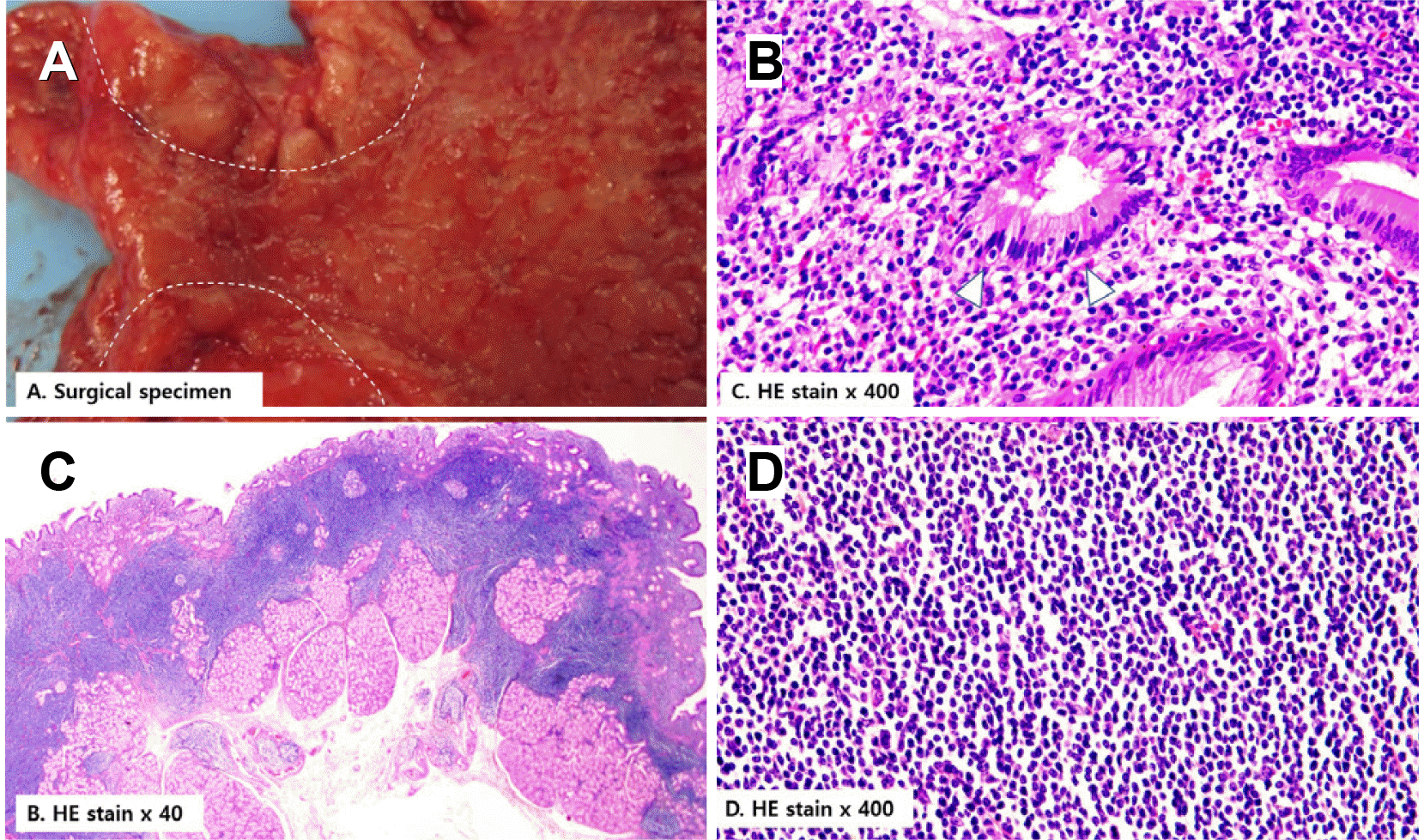
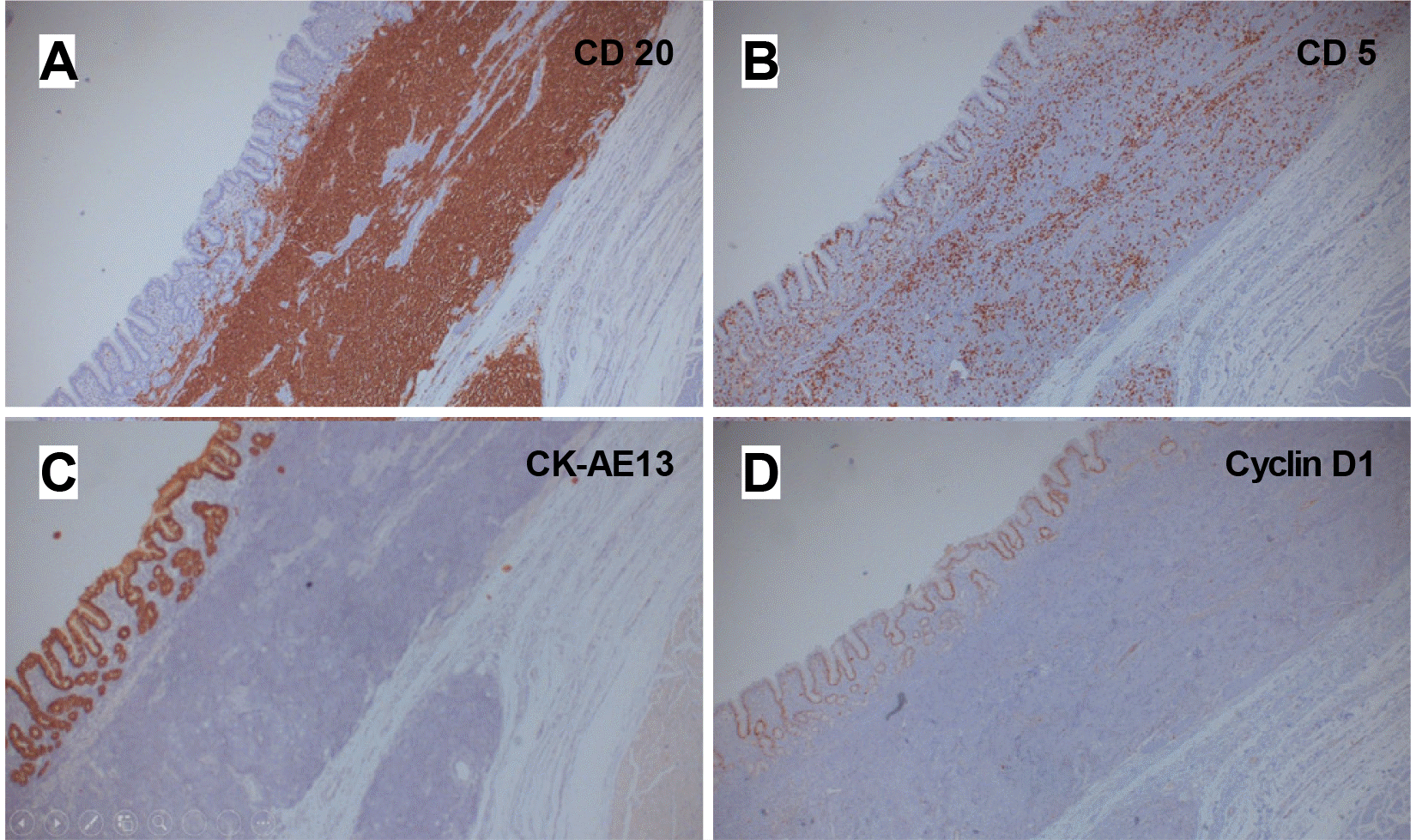
Three years later, she visited the authors’ clinic complaining of a loss of appetite. The laboratory study results were as follows: the WBC was 19,090/mm3, and the differential count for segment neutrophils was 82.1%. The hemoglobin level was 11.7 g/dL, and the C-reactive protein level was 54.86 mg/L (reference value <5.0 mg/L). The blood urea nitrogen and creatinine levels were 42.7 mg/dL and 1.36 mg/dL, respectively. On EGD, antral gastric ulcers showed geographic margins with nodular bases (Fig. 1C). The antrum and pylorus were deformed and narrowed because of the mass-forming effect of the gastric ulcers. Food and fluidic materials filling the gastric lumen suggested a gastric outlet obstruction (GOO). A contrast-enhanced CT scan and PET scan showed full layered encircling wall thickening with FDG-avid high in the antrum and several enlarged mesenteric lymph nodes, indicative of the progression of known gastric MALT lymphoma at Ann Arbor stage II1E (Figs. 2, 3). Hypotonic duodenography showed pyloric narrowing with slow contrast passage (Fig. 4). The main findings in the histological examination of the endoscopic forceps biopsy specimen were ulcer-induced debris and chronic active inflammation. Based on the results of radiologic studies and histological diagnosis two years earlier, surgery was chosen to solve the patient's gastric outlet closure. A laparoscopic distal gastrectomy with a D1 lymph node dissection and partial omentectomy was performed (Fig. 5). A gross examination of the resected distal stomach showed that the wall of the pylorus and antrum had diffuse thickening with an irregularly nodular and ulcerative mucosal surface (Fig. 5A). In a low-magnification examination, densely stained monotonous small-cell nests infiltrated the submucosal layer (Fig. 5B). Fifty-two lymph nodes were identified from the surgical sample, but no lymphoma was involved. The pathological stage was confirmed as I1E. The high-magnification examination was compatible with small lymphoid cell aggregation and lymphoepithelial lesions in the glandular epithelium (Fig. 5C, D). Immunochemical staining showed that the tumor cells stained positive with CD20, CD5 (focally positive), and PAX-5 and negative for CD3, CD10, MUM-1, and cyclin D1. The positive staining in CK AE1/3 confirmed the presence of a lymphoepithelial lesion (Fig. 6). The H. pylori infection was negative. After molecular analysis of the specimen, the presence of translocation t(11;18) was confirmed in a genetic study. No measurable lesions remained after surgery, and the treatment result was complete remission. CT scans were performed at three to six-month intervals to confirm the absence of a recurrence after surgery. In case of recurrence, involved-site radiation therapy or rituximab monotherapy was planned according to the range of lymphoma recurrence. No recurrence was observed during the last five years of follow- up, and remission was maintained.
A gastric MALT lymphoma is most often diagnosed in asymptomatic periods, and some progressive lymphoma can be accompanied by symptoms of bleeding or anemia caused by ulcers. To the best of the authors' knowledge, the symptoms of gastric outlet obstruction rarely become apparent, making it difficult to find similar cases in the literature. Gastric MALT lymphomas are treated with radiation therapy or chemotherapy for H. pylori eradication, depending on the stage. On the other hand, a surgical resection is required if there are symptoms of gastrointestinal obstruction because of the mass effect, severe bleeding from ulcers, or risk of perforation. The authors' case was a gastric MALT lymphoma accompanied by a t(11:18) mutation with a clinical course of progression despite H. pylori eradication. Despite this, tumor infiltration was limited to the submucosal layer (Ann Arbor stage I1E), and remission was maintained for five years following a surgical resection without additional treatment.
A gastric MALT lymphoma is usually indolent and responds well to standard therapy for H. pylori eradication, particularly at an early stage. A systemic review of 1,408 patients diagnosed with low-grade stage I or stage II lymphoma showed that remission occurred in 77.5%.5 Despite this, approximately 25% of gastric MALT lymphoma are refractory to H. pylori eradication.6 The optimal management of these refractory patients has not been determined, but several commonly known regional guidelines and expert opinions exist. There are several known predictors of the response to eradication therapy in patients with low-grade gastric MALT lymphoma: a H. pylori infection; Lugano classification stage I; lymphoma confined to the stomach; gastric wall invasion limited to the mucosa or submucosa; and the absence of gene t (11, 18) (q21; q21), translocation with the fusion of API2 and MALT1.7
Management strategies vary widely from ‘watch-and-wait’ to chemotherapy, radiotherapy, and immunotherapy, such as rituximab. Considering that no consensus has been established, the usual approach with patients without progressive disease is to use the ‘watch-and-wait’ method for up to 24 months, while radiotherapy and chemotherapy are usually performed for advanced local disease. The clinical outcome data slightly favor radiotherapy in terms of its effectiveness. Chemotherapy and rituximab are usually reserved for more disseminated diseases or cases with a histological transformation to a more aggressive form. Surgery is usually reserved for cases with complications, such as perforations, stricture, or bleeding.8,9
The patient was followed up with endoscopy for 17 months after being diagnosed with MALT lymphoma and following a subsequent H. pylori eradication, which did not appear to progress at the time. After another 16 months, it developed aggressively into a full-blown advanced malignant lymphoma with a gastric outlet obstruction. This paper reports a rare presentation of gastric MALT lymphoma.
REFERENCES
1. Isaacson P, Wright DH. 1983; Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 52:1410–1416. DOI: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3. PMID: 6193858.

2. Wang YG, Zhao LY, Liu CQ, et al. 2016; Clinical characteristics and prognostic factors of primary gastric lymphoma: A retrospective study with 165 cases. Medicine (Baltimore). 95:e4250. DOI: 10.1097/MD.0000000000004250. PMID: 27495029. PMCID: PMC4979783.
3. Zaki M, Schubert ML. 1995; Helicobacter pylori and gastric lymphoma. Gastroenterology. 108:610–612. DOI: 10.1016/0016-5085(95)90098-5.

4. Park HS, Kim YJ, Yang WI, Suh CO, Lee YC. 2010; Treatment outcome of localized Helicobacter pylori-negative low-grade gastric MALT lymphoma. World J Gastroenterol. 16:2158–2562. DOI: 10.3748/wjg.v16.i17.2158. PMID: 20440857. PMCID: PMC2864842.

5. Zullo A, Hassan C, Cristofari F, et al. 2010; Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol. 8:105–110. DOI: 10.1016/j.cgh.2009.07.017. PMID: 19631287.

6. Kim JS, Park JC, Lee JY, et al. 2021; Long-term clinical outcomes of gastric MALT lymphoma: A nationwide multicenter study in Korea. Front Oncol. 11:681689. DOI: 10.3389/fonc.2021.681689. PMID: 34722238. PMCID: PMC8551628.

7. Malfertheiner P, Megraud F, O'Morain C, et al. 2007; Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 56:772–781. DOI: 10.1136/gut.2006.101634. PMID: 17170018. PMCID: PMC1954853.

8. Nakamura S, Hojo M. 2022; Diagnosis and treatment for gastric mucosa-associated lymphoid tissue (MALT) lymphoma. J Clin Med. 12:120. DOI: 10.3390/jcm12010120. PMID: 36614921. PMCID: PMC9820981.

9. Moleiro J, Ferreira S, Lage P, Dias Pereira A. 2016; Gastric malt lymphoma: Analysis of a series of consecutive patients over 20 years. United European Gastroenterol J. 4:395–402. DOI: 10.1177/2050640615612934. PMID: 27403306. PMCID: PMC4924435.

Fig. 1
EGD findings on each patient’s visit. (A) Multiple gastric and duodenal ulcers with pyloric deformity. (B) Several gastric ulcers at the antrum and lower body, and duodenal ulcers at various stages. Histology of one ulcer (indicated) confirmed as marginal zone MALT lymphoma. (C) Pylorus deformity and luminal narrowing due to mass forming effect of geographic and speculated ulcers. EGD, esophagogastroduodenoscopy; MALT, mucosa-associated lymphoid tissue.
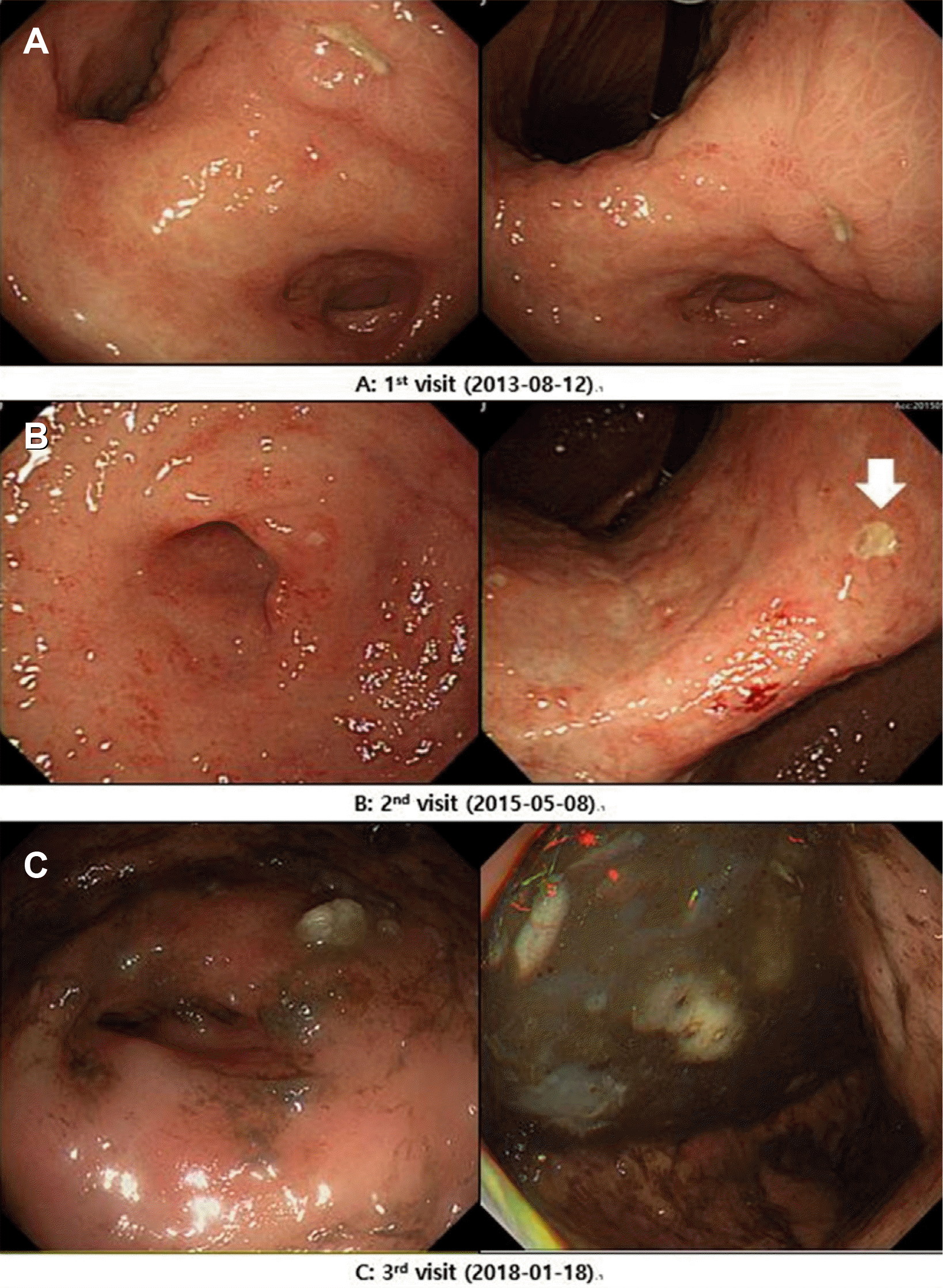
Fig. 2
CT scan of the abdomen, coronal plane. Poorly enhancing encircling mass in stomach antrum with ulceroinfiltrative feature corresponds to gastric outlet obstruction due to advanced malignancy.
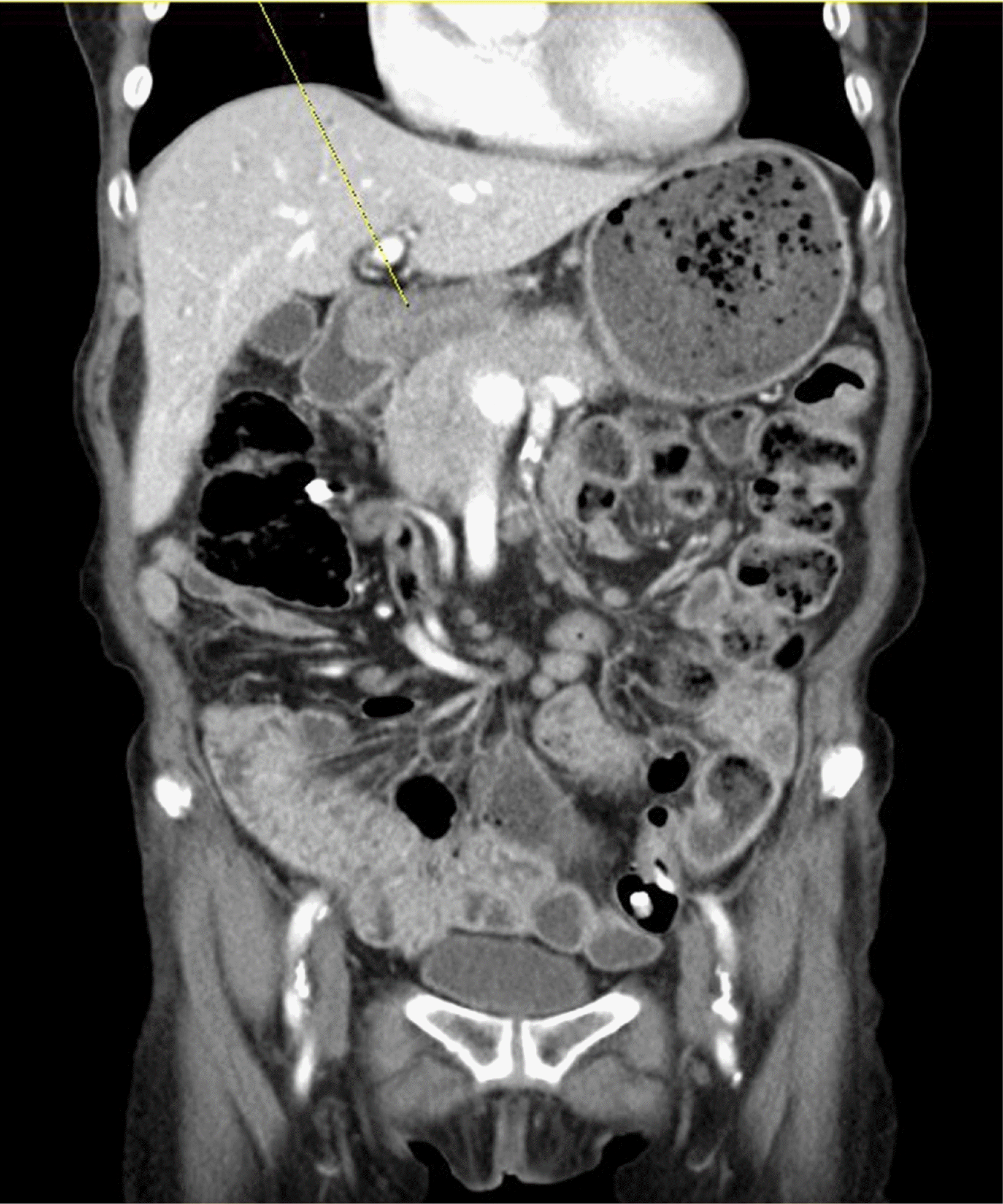
Fig. 3
PET scan. PET scan showed a full-layered encircling wall thickening with FDG-avid high in the antrum and several enlarged mesenteric lymph nodes, suggesting a gastric malignancy.
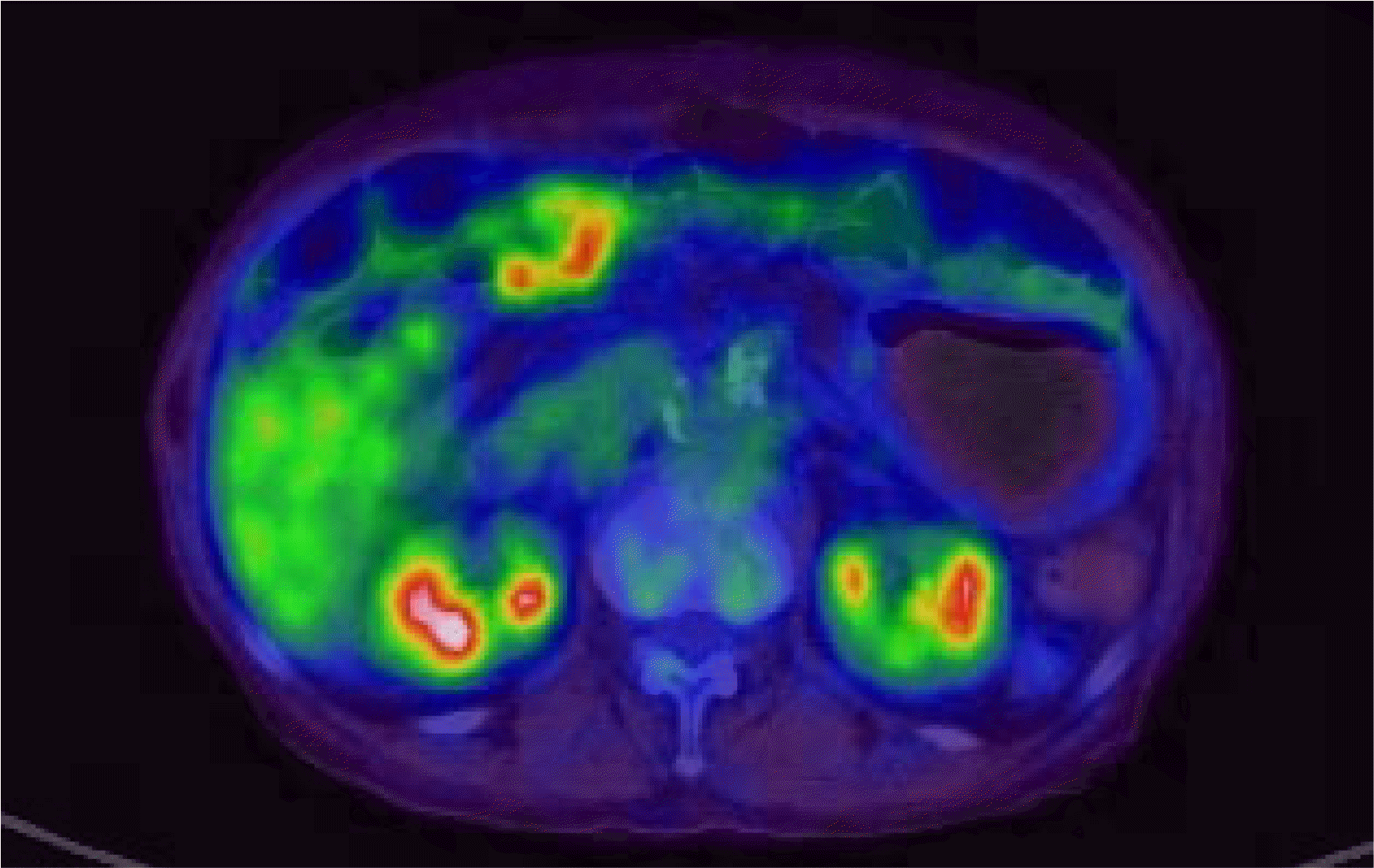
Fig. 4
Hypotonic duodenography. Duodenography under antispasmodic agent showed pyloric narrowing with slow contrast passage. Arrows: narrowed pyloric canal.
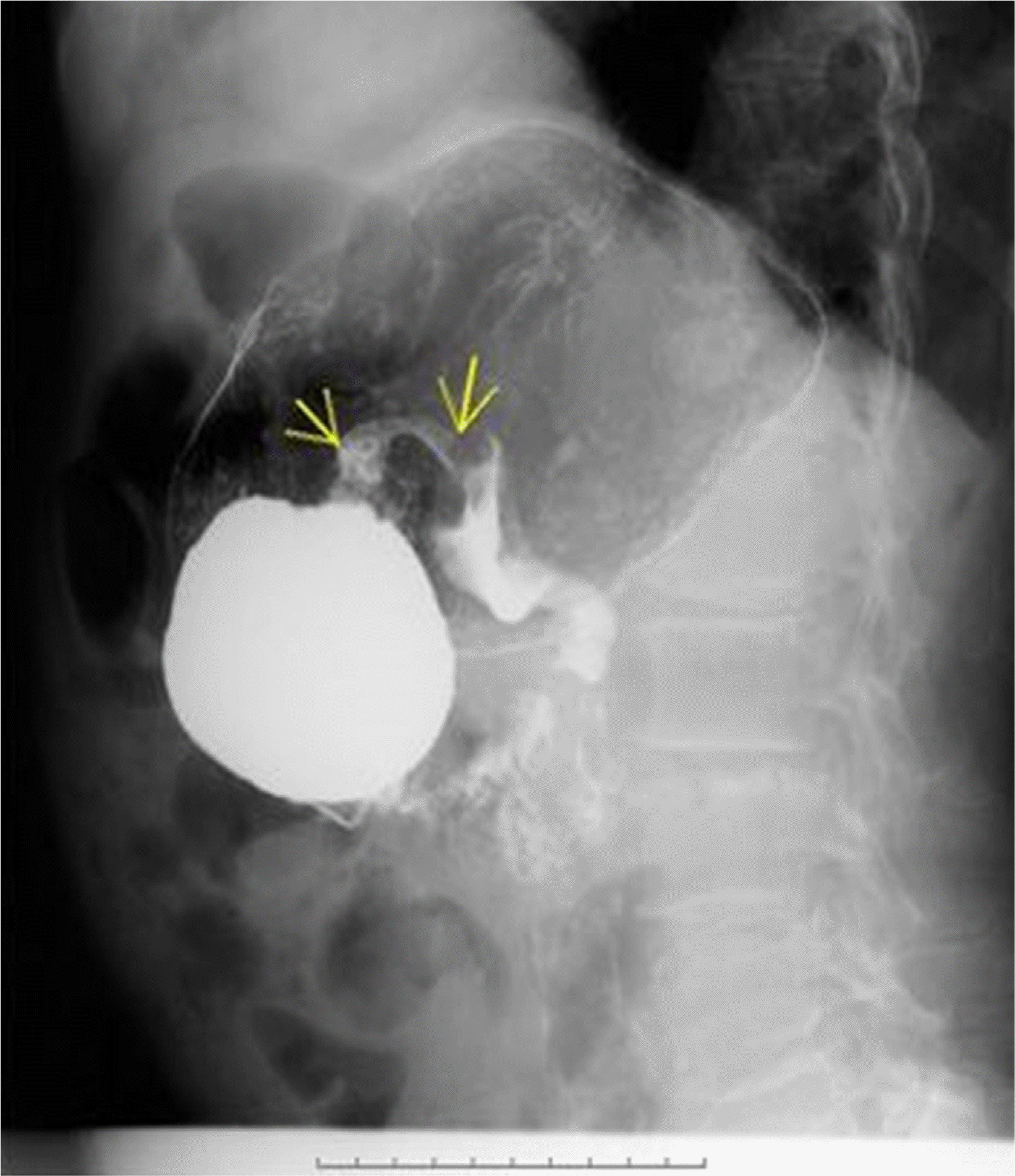
Fig. 5
Gross finding of surgical specimen and microscopic examination of H&E staining. (A) The wall of the pylorus (white dotted line) and antrum showed diffuse thickening with the irregularly nodular and ulcerative mucosal surface. (B) Microscopic image (H&E stained ×400) showed lymphoepithelial lesions in the glandular epithelium (C, white arrowheads). (C) Macroscopic image (H&E stained ×40) showed diffuse and monotonous small lymphoid cell infiltration formed tumor mass. (D) Microscopic image (H&E stained ×400) showed densely stained monotonous small lymphoid cell infiltration into the submucosal layer.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download