Abstract
Background
Advances in neuroscience and neurotechnology provide great benefits to humans though unknown challenges may arise. We should address these challenges using new standards as well as existing ones. Novel standards should include ethical, legal, and social aspects which would be appropriate for advancing neuroscience and technology. Therefore, the Korea Neuroethics Guidelines were developed by stakeholders related to neuroscience and neurotechnology, including experts, policy makers, and the public in the Republic of Korea.
Method
The guidelines were drafted by neuroethics experts, were disclosed at a public hearing, and were subsequently revised by opinions of various stakeholders.
Results
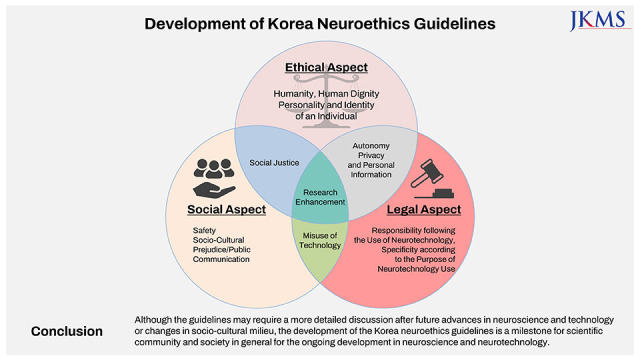
The guidelines are composed of twelve issues; humanity or human dignity, individual personality and identity, social justice, safety, sociocultural prejudice and public communication, misuse of technology, responsibility for the use of neuroscience and technology, specificity according to the purpose of using neurotechnology, autonomy, privacy and personal information, research, and enhancement.
Conclusion
Although the guidelines may require a more detailed discussion after future advances in neuroscience and technology or changes in socio-cultural milieu, the development of the Korea Neuroethics Guidelines is a milestone for the scientific community and society in general for the ongoing development in neuroscience and neurotechnology.
Graphical Abstract
Neuroscience and neurotechnology are developing rapidly, as a result, the functions of the brain are being revealed. Neuroscience refers to a scientific approach that pursues a systematic understanding of the structure and function of the nervous system in humans and animals, and neurotechnology is a technology to observe, evaluate, access, and control the nervous system based on this scientific understanding. ‘Neuroscience’ is the study of molecules, cells, development, structure, function, evolution, computer connections, and medical aspects of all nervous systems including the brain. ‘Neurotechnology’ includes fields such as brain nervous system imaging, neuromodulation technology, brain-machine interface (BMI), and collection, storage, and processing of neural information or related information. Discovering the underlying principles of the brain functions is the ever-seeking goal of the majority of neuroscientists. Such innovative advances in neuroscience and neurotechnology are expected to result in interventions that have previously been impossible in human disease treatment and health promotion. The understanding of human beings must be re-evaluated via new facts regarding important aspects, including the relationships between thoughts, emotions, and behaviors, as well as personal and socio-cultural perceptions. Neuroscience and neurotechnology research and development provide significant benefits to society and individuals, and large-scale investments are being made at the national level for this purpose.
In the light of the investment, development, and impact of neuroscience and technology, new issues and considerations must be addressed. The field of neuroethics, which includes the Responsible Research and Innovation (RRI) and Ethical, Legal, and Social Implication (ELSI) approaches, is essential to address these new issues.123 The advancement of neuroscience and neurotechnology and application of these novel developments to patients or research subjects requires new ethical, legal, and social discussions that have not yet been addressed. These issues are of important significance for individuals and society as a whole.
The basic principles of bioethics that humankind must follow at an international level have been declared in the 1947 Nuremberg Code, the 1964 Declaration of Helsinki adopted by the World Medical Association, and the 2005 “Universal Declaration on Bioethics and Human Rights” by the United Nations Educational, Scientific, and Cultural Organization. The status of recently published research regarding neuroethics, guidelines, and reports is presented below.
From 2006 to 2020, approximately 22 neuroethics guidelines and reports have been published targeting various stakeholders including researchers, research volunteers, medical staff, professional institutions, and policy makers with the participation of international organizations such as the Organisation for Economic Co-operation and Development; countries including the European Union, the United States, United Kingdom, and Japan; and various private organizations.4 The neuroethical recommendations presented in these guidelines and reports focused on ethical, legal, and social issues and were published regarding the entire field of neuroscience and technology. Guidelines and recommendations regarding specific neurotechnologies (such as non-invasive neuromodulation and brain-machine interfaces) have also been published. The publications discuss autonomy, identity, informed consent and capacity, privacy and data protection, governance, patient selection criteria, justice and equity, legal responsibility, assessments of safety and efficacy, medical and non-medical uses, neuro-economics, enhancement, and research.
Since the 1998 enactment of the “Brain Research Promotion Act” in Korea, research has been conducted at a national level with the establishment of the “First Basic Plan for Brain Research Promotion” in 1999, the “Second Basic Plan for Brain Research Promotion” in 2007, and the “Third Basic Plan for Brain Research Promotion” in 2018. According to Articles 5 and 6 of the Brain Research Promotion Act, related ministries such as the Ministry of Science and ICT; the Ministry of Education; the Ministry of Trade, Industry, and Energy; and the Ministry of Health and Welfare must establish and implement the ‘Brain Research Promotion Implementation Plan’ every year. In 2009, the Korea Brain Research Institute was established based on the same law, and the national brain research strategy policy was established by the Brain Research Policy Center affiliated with the institute. This institute plays an active role in the field of international neuroethics and hosted the Global Neuroethics Summit (GNS) in Korea in 2017, 2018, and 2019. The GNS is an event hosted by the International Brain Initiative (IBI). Several countries worldwide participate in the IBI and GNS.
The discussion regarding neuroethics requires international exchanges and cooperation. The most prominent global activities regarding neuroethics include the brain neuroethics consortium of the Global Neuroethics Working Group of the International Brain Initiative. The working group discussions include strengthening the integration and cooperation between neuroscience and neuroethics, which is continuously researched by experts.5
With continuous research and applications of neuroscience and neurotechnology, novel challenges regarding personality, autonomy, enhancement, and privacy protection may arise. While existing ethical standards may be useful to overcome these challenges, new ethical, legal, and social standards may be needed at times. These challenges must be addressed with the participation of neuroscientists, medical practitioners, ethicists, philosophers, sociologists, jurists, lawyers, and other stakeholders. To achieve responsible innovation during this process, cooperation between the government and public and private sectors is required. An open discussion of the best blueprint for the future based on advancements in neuroscience and neurotechnology can then occur.
However, existing international guidelines are generally too abstract or limited to specific technologies. So, it is necessary to examine the characteristics of neuroscience and neurotechnology more sensitively in ethical, legal, and social aspects. As part of this effort, the establishment of the Korea Neuroethics Guidelines was achieved with the participation of researchers, experts, and the general public with government research support.
The development of the Korea Neuroethics Guidelines employs ELSI methodology based on a comprehensive literature review and analysis of existing guidelines, relevant laws and regulations, scientific articles, and expert reports.6 The ELSI methodology began with the Human Genome Project and was established as one of the most standard approaches to assessing and preparing the impact of novel science and technology on individuals and society at large. The guidelines development was initiated by a number of neuroethics experts affiliated with Neuroethics Research Society under the Korean Bioethics Association, and they created a guidelines development team in October 2020. The team was composed of neuroethics experts with backgrounds in the fields of medicine, neuroscience, law, and ethics.
The guidelines were established through the following process. For the literature review, existing neuroethics guidelines, major guidelines concerning biomedical ethics and research ethics, relevant laws and regulations, scientific articles regarding advancing neuroscience and neurotechnology78910 and expert reports on various neuroethical problems were searched, collected, sorted out, and classified according to the time of publication, addressing stakeholders, guidelines authors, and the kinds of technology.11 With these classifications the team identified more than twenty neuroethical issues which were mentioned and discussed repeatedly in the literature review. Based on the criteria of significance and timeliness of the issue, and possibility and potential severity of human impact, all those issues were analyzed, and twelve issues were finally selected. Then the team coded the issues according to the ethical, legal, social and mixed aspects. Issues related to humanity, human dignity, personality and identity of an individual were included in ethical aspects. Issues related to legal responsibility and issues related to safety, social prejudice and public communication were included in legal and social aspects respectively. Those issues concerning multiple aspects were included in mixed aspects.
Based on these review and analysis the draft of the guidelines was prepared by the team in December 2020. The draft was constantly revised by the team members until the disclosure at a public hearing in September 2021. The draft was circulated among neuroethics experts associated with the Neuroethics Research Society under the Korean Bioethics Association, and some of them returned their comments. For the public hearing at least one representative from each of the fields of neuroscience, medicine, law, and ethics was invited and actually participated. It was organized by the guidelines development team and was sponsored by the National Research Foundation of Korea and the Korean Institute of Criminology and Justice. Participants of the public hearing were diverse including neuroscience researchers, medical doctors, nurses, neuroethics experts, biomedical and research ethics experts, medical educators, opinion leaders in the fields of medicine and professional academic associations, and lay people. All the representatives from various fields stated their concerns with the draft in the hearing. Their common concerns were the vagueness or strictness of the guidelines draft. Some parts of the guidelines were not clear enough for definite action guidance in specific situations and some parts of them were too strict that it might dampen neuroscience researchers’ spirits and thwart neuroscience research all together. The draft was thoroughly revised according to these concerns and other suggestions from the representatives and participants of the hearing. The guidelines were finally confirmed by the guidelines development team with the aforementioned criteria, and were made public in July 2022.
The neuroethics guidelines were classified as ethical, legal, or social aspects, and overlapping items included in each aspect were determined (Fig. 1). Whether each issue is more of an ethical, legal, or social aspect is only a schematic distinction as no issue truly involves only one aspect. Nevertheless, for the convenience of discussion, the issues were tentatively classified based on the weight of major concern for each issue.
The target audience of the guidelines is experts or institutions including researchers, neurotechnology developers, and medical personnel in the neuroscience and neurotechnology fields (collectively referred to as “experts”), corporate decision makers, companies, policy makers, and regulatory authorities. The end users of the guidelines may include patients and general consumers, though the term “users” in these guidelines refers to medical personnel or professionals who apply neurotechnology to patients.
New science and technology developments create new possibilities, hopes, and a reality that did not previously exist, though challenges are also created. Some challenges are unpredictable. The brain is a major organ that controls human cognition, emotion, will, behavior, and consciousness, which are the main characteristics of humanity. If the development of neuroscience and neurotechnology affects them, it must be carefully considered and accessed. Furthermore, novel neurotechnology may affect these characteristics directly and more powerfully than existing technologies.121314 Human cognition and emotion can be changed via education and public opinion. However, if neuroscience and neurotechnology can directly improve human cognition or alter emotions in a positive manner over a short period of time, these changes would differ from existing methods of affecting cognition and emotion. Therefore, the impact of neuroscience and neurotechnology on humanity should be reviewed and the level and extent of the impact should be evaluated.
In addition, advances in neuroscience and neurotechnology may affect human dignity. As rationality or autonomy, which is typically presented as the basis of human dignity, presupposes appropriate cognition, emotion, will, and behavior, factors affecting these functions may also lead to changes in rationality or autonomy. Therefore, the main characteristics of changes in rationality or autonomy occurred due to neuroscience and neurotechnology as well as the impact of these changes on individuals and society should be examined in detail.
However, humanity and human dignity have different meanings in different societies and cultures. Therefore, if neuroscience and neurotechnology can influence the existing concepts and understanding of humanity or human dignity, socio-cultural conflicts or conflicts of interest may arise. Thus, communication procedures should be established to properly mediate such conflicts.
If newly developed neurotechnology results in obsessive thoughts or behaviors that one cannot control or in different emotional states, it influences an individual’s identity. In addition to identity, neurotechnology can influence an individual's ability to act or her/his subjective experience and perception of the surrounding environment.1516 Neurotechnology can affect an individual’s identity and agency by specifically affecting individual cognition, emotion, memory, and will. Therefore, new theoretical approaches regarding an individual’s identity and ability to act may be needed. A fundamental reflection of the definition of personal identity and agency may be also necessary.
When an individual’s identity or ability to act is affected by neurotechnology, whether or not their identity or agency should be recognized as their own identity or agency should be determined.17 When the individual identity is formed using neurotechnology, it is unclear whether treatment or consumption decisions made by the individual can be recognized as their own decisions.
Therefore, when neurotechnology to be applied to patients or research subjects, experts must provide adequate and sufficient information regarding the overall effects, including the impact of the neurotechnology on an individual’s identity, and research subjects or patients should decide whether to use the neurotechnology after understanding the overall short- and long-term effects. If the neurotechnology affects an individual’s identity or ability to act, a consent procedure to provide relevant information should be prepared in advance.
In addition, experts must objectively evaluate the effect of new technologies on personal identity according to professional conscience. If the effect is estimated to be significant, the neurotechnology must be re-evaluated according to a pre-established procedure. Experts must confirm the effects of neurotechnology on an individual’s identity or behavioral ability via empirical research. If a specific neurotechnology affects human identity, regulatory authorities and relevant industries must adopt the licensing of the neurotechnology or consider its commercialization with caution.
Advances in neuroscience and neurotechnology renew the understanding of nervous system diseases and symptoms. However, these advances may also present unpredictable risks to research subjects and patients. Therefore, when using neuroscience and neurotechnology on human subjects, safety must be given priority.10 When new neurotechnology is used on humans, the risks and long and short-term effects must be considered.18
Ensuring the physical, psychological, and emotional safety of human subjects in neuroscience research and during the use of novel technologies must have the top priority. A thorough investigation and review of safety, focusing on short- and long-term side effects should be conducted, and safety evaluation standards should be prepared. As the long-term side effects and risks are unclear in using novel devices, sufficient research must be conducted prior to their use.19
The safety of novel neuroscience and neurotechnology should be evaluated in terms of foreseeable benefits and risks. If their risk is low, they can be considered to be relatively safe, but their long-term effect should always continue to be reviewed. This should not be interpreted as an unconditional ban on the use of new neuroscientific methods and technologies; instead, it should enable society to continuously contemplate and discuss criteria that can allow or promote neuroscience and technology.20
During the development of neuroscience and technology, pre-existing sociocultural prejudices may affect the understanding and interpretation of new advances. Incomplete information generated during the early stages of neuroscience research may create new social prejudices or deepen existing social biases. Therefore, the use of new neuroscientific discoveries and technologies should avoid methods that result in negative evaluation, prejudice, or social stigmas to specific individuals or groups. In addition, existing socio-cultural prejudices cannot be used to interpret novel neuroscientific findings or to use new technology.
Neuroscience and neurotechnology researchers must explain and promote their research achievements within a scientifically acceptable range. Care must be taken not to cause misunderstandings and to avoid errors in over-generalization when communicating with the public regarding new advances.
The public’s understanding of neuroscience and neurotechnology is a critical component in supporting neuroscience research and neurotechnology development. To help the public understand novel advances, education programs with citizen participation should be prepared.21 A communication system should be also established to discuss topics related to neuroscience and neurotechnology at any time.
The media significantly influences the public's understanding of neuroscience and neurotechnology.22 Therefore, relevant experts should not give advice to the media in a way that may cause distortion or exaggeration. Researchers should not unreasonably advertise their achievements via the media. Furthermore, the media is responsible for reporting the achievements in neuroscience and neurotechnology without distortion or exaggeration.
As the understanding of brain functions becomes more detailed with new advances, social and cultural prejudices should be avoided by considering neurodiversity.23 Brain functions that are considered to be so called ‘normal’ may vary; therefore, normal brain function should not be considered to be fixed as well as brain function that deviates from normal should not be considered to be abnormal. These behaviors should be avoided from the stage of research planning through that of interpreting the results to that of applying technology.24
Prior to discussing the issue of liability associated with the use of neurotechnology, it is necessary to discuss whether neuroscience and neurotechnology are changing the notion of liability. The basic framework and requirements of the normative concept of legal responsibility will not change with the development of neuroscience and technology. However, neuroscience and neurotechnology can be used as strong evidence in the process of normative judgment for attributing responsibility, and the specific criteria for judgment should be reviewed continuously.
The effects of neuroscientific performance and neurotechnology on patients, consumers, and research subjects should be reviewed in advance of the use of novel technology. Harm to patients, consumers, and research subjects should be avoided. Detailed investigations are, therefore, required and then appropriate actions should be carried out if harm occurs.
Various parties are responsible for problems that occur with the use of neurotechnology, including the researchers, developers, and manufacturers of the technology (vendors, medical personnel, medical institutions, consumers, patients, and the state). Therefore, it is necessary to identify the results of the use of novel neuroscience methods and neurotechnologies, to prove a causal relationship, and to clarify the responsibility in detail. The assignment of responsibility must be thoroughly examined in each case. It is necessary to consider all relevant parties as a responsible agent and to review whether each of them is responsible for each act. In some events, the state may be liable for compensation.25
However, even when responsibility is assigned to each agent, a causal relationship, which is needed to impose responsibility, may be difficult for the patient or customer to prove, due to their lack of information or expertise. If the responsibility does not lie with a patient or a consumer, it is necessary to consider a system that relieves the proof burden of a causal relationship or proves it from their perspective, so that they may not be harmed due to others’ evasion of responsibility as well as in the process of identifying the responsible party.26
Apart from the clear identification of responsibility, if the responsible party or the scope of liability for side effects and damage is too large, it can adversely affect the motivation of those who want to develop and use neurotechnology. To prevent a defensive attitude of the user, a new insurance system for unexpected damage or side effects should be developed.27 In addition, it is necessary to establish a system and procedure that can prevent and manage problems before such problems arise.
Neurotechnology can be used for a variety of purposes, including medical, commercial, military, and legal purposes. According to each purpose, it is necessary to review and confirm the facts and conditions to be considered prior to the use of neurotechnology.
The use of neurotechnology for medical purposes may or may not be a standard treatment. When a certain technology used as the standard treatment, medical personnel must faithfully fulfill their duty of explaining the use of the neurotechnology, obtaining informed consent based on the patient’s full understanding, and paying close attention to the medical practice and to the use and management of medical devices. In addition to the opinions of experts of the relevant medical fields, the socioeconomic and psychological aspects of the use of neurotechnology should also be considered, and, furthermore, an integrated and collaborative approach should be promoted. When the neurotechnology does not correspond to the standard medical treatment and is used for humanitarian purposes, medical personnel must continue to explain its use to the patient, obtain an informed consent, and pay attention to the use and management of the medical device. Especially for desperate patients having no other options, treatment should not be attempted indiscriminately without considering the harm to them.282930
When neurotechnology is used for commercial purposes, developers, manufacturers, and vendors should not exaggerate the safety and efficacy of the applied technology. Appropriate regulations and management are required against exaggerations supported by manufacturers or sellers. In this case, if neurotechnology is used non-medically, the medical-related regulations are not applied, and the general consumer product-related regulations may be applied. However, it may be treated to be similar to the case of medical use, as neurotechnology specifically affects the brain and nerves.83132
The military use of neurotechnology must follow specific and clear regulations, and a management system and procedure must also be established. When conducting research or using neurotechnology that contains elements that violate human rights, a legal system to prohibit such use should be established.
When neurotechnology is used for correctional purposes, several prerequisites regarding other correction methods must be met. Neurotechnology used for correctional purposes must not be irreversible and its efficacy must be scientifically proven. The use of neurotechnology must be imposed on criminals via a just and due process. In addition, as the purpose of such use of neurotechnology would be to prevent re-offenders and to correct criminals, these uses must comply with the principle of proportionality in legislation and enforcement.33
After the use of neurotechnology for any purpose, records must be prepared and kept, side effects must be tracked and monitored, and personal information must be managed and protected. A management and supervision system should be prepared for the alleviation of side effects and for the protection of personal information.
If advances in neuroscience and neurotechnology affect higher mental functions such as cognition, emotion, and will, the impact of such developments must be examined. When new technologies affect cognition, emotion, and will, an individual's autonomy and decision-making ability may also be affected.34 In addition, the concept of autonomy is not fixed, but can be understood and interpreted differently depending on the characteristics and context of the society and the interactions between its members. For example, some elderly patients in Korea often allow family members to make medical decisions. While these patients may have given up their right to make decisions, they may be viewed as expressing their desire to make a decision in consultation with their family. Therefore, as neuroscience and neurotechnology continue to advance, a fundamental approach and review regarding the form and content of individual autonomy is necessary.
When applying new neurotechnology to humans for research purposes or applying commercialized neurotechnology for treatment, informed consent must be obtained. The application of the novel neurotechnology should be a voluntary decision made by the patient. When neurotechnology to be applied to a research subject or patient, an expert must provide appropriate and sufficient information regarding the effects of the technology, and the research subject or patient must understand the overall impact including potential short- and long-term effects prior to the application of the technology. Research subjects or patients should be allowed to withdraw from research participation or treatment at any time, even if the study or treatment are ongoing.
Experts should carefully evaluate the ability of research subjects or patients to consent if they are unable to communicate, are minors, or have reduced decision-making ability. Decision-making ability should not be assessed solely on the basis of pre-existing diseases or temporary conditions. Instead, decision-making ability should be evaluated in a variety of ways using methods that optimize the ability rather than via a single evaluation. If the ability of the research subject or patient to consent is clearly impaired, sufficient information and support should be provided to her/his family members or legal representatives so an appropriate proxy decision can be made.
Experts should assure that the use of neuroscience and neurotechnology is voluntarily chosen by the consumer, not coerced by others, businesses, or communities. If the solicitation of another person, company, or community is voluntary, the use of the neurotechnology is acceptable. However, the influence of solicitation regarding the use of new neurotechnology on the individual's autonomous decision should be carefully reviewed.
Relevant experts and scholars should identify and discuss ethical, legal, and social issues and issues that may arise from neurotechnology that affect individual autonomy. In addition, a response system based on a social consensus should be established. Government agencies should also cooperate one another in these efforts.
Devices that measure the neuronal activity of the brain or stimulate specific areas of the brain are currently in use. There are efforts to achieve effective brain stimulation by monitoring pathological biomarker signal in the brain.35 However, with further advances in neuroscience, the abilities to induce a change in an individual’s specific cognition, emotion, or will may be possible by applying brain stimulation for clinical and commercial uses.36 The use of these technologies for personal purposes or corporate commercial ones or for national interest like surveillance purposes will be highly controversial.
Therefore, if an individual does not want his or her mental state to be accessed, a system and a method to properly protect neuroprivacy should be developed to deny such access. Neuroprivacy refers to the right to deny unauthorized access to an individual's neural information.37 However, these general regulations alone cannot constitute a system and a method that can adequately protect neuroprivacy; a detailed plan regarding how an individual’s neuroprivacy can be protected is needed.
Human beings benefit greatly from the development of novel devices and procedures, though care must be taken not to allow undue invasion or undue influence on individuals, especially undue invasion of personal privacy and confidentiality. When an individual discloses his/her personal information at his/her own will, this information must be protected appropriately. The principle of personal information protection must be strictly enforced, even when personal information is obtained for secondary use.
Furthermore, if neurotechnology can be used to measure and interpret an individual’s brain signals without the individual’s awareness, it will be necessary to thoroughly review the concepts of privacy and protection of privacy, which were established on the premise that access or interference from others can be controlled. As information collected from the brain may be used by others before the owner of such information can determine the content and scope of the information disclosed, it is necessary to reconsider the content and scope of the right to make decisions regarding personal information. It is also necessary to discuss whether advances in neuroscience and neurotechnology require additional rights regarding privacy and personal information protection.
With future advancements in neuroscience and neurotechnology, the right to make one’s own decisions regarding neuroprivacy and neural information protection will emerge as an important issue. A new set of rights may emerge in relation to neural information. Therefore, it is necessary to understand the ethical, legal, and social issues related to neuroprivacy and neuroinformation and to prepare a response system based on the consensus reached via a discussion with members of society.
While advances in neuroscience and neurotechnology may create new inequalities or exacerbate existing ones, they may also alleviate existing inequalities. Problems of social justice that can be brought on by neuroscience and neurotechnology may lead to both negative and positive outcomes in our society; therefore, a balanced approach and consideration are needed. If human functions can be augmented using neurotechnology and only certain socioeconomic classes receive the benefits of these technologies, it will lead to social inequality.38
As such, neuroscience and neurotechnology can be positive or negative depending on the purpose, procedure, or standard for which they are used within a society. Conducting research or using neurotechnology that may result in disadvantages such as social prejudice, stigma, or discrimination should be avoided.
In addition, when neuroscience research and neurotechnology developments are conducted using public funds, the outcomes and the uses of such research and neurotechnology must be public in their nature. Therefore, neurotechnology developed using public funds should be fairly accessible to all members of society, and the researcher’s efforts should be evaluated fairly.
Neurotechnology has the potential to be used for any purposes other than its intended use (neuroscience and technology developed for medical purposes can be used for commercial or military purposes; neurotechnology developed for commercial purposes could also be used for military purposes), though this should be avoided as much as possible. The misuse of neurotechnology must also be avoided. Prior to the use of neurotechnology, it should be verified that no human rights will be violated. In particular, the use of neurotechnology for military purposes must be limited with careful consideration of its misuse or abuse. A management and monitoring system must be established at the national and international level.24
Neuroscience research and neurotechnology development are sometimes accompanied with human subjects or human driven materials. During the research process, an understanding of ethical principles and legal regulations regarding obtaining informed consent, the protection of personal information, and the secondary use of collected information is essential. Therefore, it is necessary to develop a system that makes such education compulsory.
With the rapid development of neuroscience and technology, existing laws and their sub-regulations cannot properly regulate research regarding some novel drugs, medical devices, and medical interventions. Therefore, the relevant laws and sub-regulations must be reorganized, which may also require changes to ethical, legal, and interdisciplinary meanings.39 Specific neuroscience research may be deemed unethical or illegal, and the requirements for acceptance must be determined. Interdisciplinary discussions may be needed at the beginning in the research planning stage so that individual or social biases may not interfere with the design and conduct of research and so interdisciplinary concepts may be communicated smoothly. In addition, various understandings and interpretations of concepts that contain the uniqueness of each society and culture must also be considered during the entire research planning and execution.
The moral status of the human brain model developed for neuroscience research, such as a brain organoid, must also be reviewed. Moral concerns should be handled appropriately.24 A new ethical standard or governance that can respond to such concerns is necessary.
Human enhancement is commonly defined as being contrast to therapy. Enhancement is the use of medical interventions (pharmacological, surgical, or biotechnological interventions) in order to enhance the physical, mental, and cognitive functions of a healthy person beyond prevention and treatment.4041 However, treatment can be flexible according to the society, culture, and era in which it is conducted, and the same medical intervention can be used for treatment or for enhancement. Therapeutic medical interventions may also result in enhancement. Therefore, it is difficult to clearly distinguish treatment from enhancement. Thus, the fluid relationship between treatment and enhancement in neuroscience research must be considered as well as the use of neurotechnology for enhancement purposes must be carefully reviewed.
Sometimes neuroscience and neurotechnology can be used for enhancement as well as treatment. When neuroscience and neurotechnology is used for the purpose of enhancement, the impact on an individual and a society should be reviewed in advance. The target for enhancement; purpose, type, and method of the neurotechnology used; conditions; expected long-term and short-term benefits and risks; and socio-economic impact must be carefully considered.28
When neuroscience and neurotechnology are used for enhancement purposes, the safety of the user may be in jeopardy, personal autonomy may be violated by forcing individuals to use specific technologies, and an access to specific technologies may be unfairly constructed. While the use of neuroscience and neurotechnology for enhancement may alleviate the problem of inequality among individuals in society,42 inequality may also be exacerbated. Furthermore, enhancement has the potential to change an individual’s identity and personality as well as the identity of the human species.4344 Therefore, the use of neuroscience and neurotechnology for the purpose of enhancement requires continuous and extensive social discussion from various perspectives.
The development of the neuroethics guidelines is significant in that they are the first neuroethics guidelines developed in Korea. Several ELSI studies have been conducted in Korea, but this was the first regarding neuroscience and technology. These guidelines will be referenced in future research and development. The development of these guidelines includes stages in which their draft is created and reviewed through consultation with interdisciplinary experts, including scientists. These guidelines can play a role as a draft guideline that can contribute to establishing guidelines by national institutions in the future. In addition, the effects of each issue of neuroscience and neurotechnology development were considered more in-depth than previously published neuroethics guidelines or reports. In these guidelines, various aspects of neuroscience research and technological development have been preemptively considered beyond what is discussed in existing bioethics guidelines. These guidelines also suggest the direction of ongoing neuroscience research and neurotechnology development, indicating the issues that should be considered. Furthermore, the ethical and legal consideration as well as principles that should be considered in neurotechnology development are presented in these guidelines, though it is difficult to achieve a specific result with the current level of technology.
Although these neuroethics guidelines have great significance, they must continue to be revised and supplemented. According to the development of neuroscience and technology, each issue presented in these guidelines should be further sub-divided to develop systematic guidelines based on real, clinical practice. It is hoped that future guidelines can be specific enough to include guidelines and systems that can be used for each novel technology. These current guidelines should also be supplemented with quantitative and qualitative empirical research. Future guidelines should refer to such research to establish a realistic basis that can be reflected in actual laws and systems. The current guidelines must be revised continuously and supplemented based on the rapidly changing research environment and culture in the Republic of Korea. In addition, these guidelines may be useful in the development of future domestic guidelines and international guidelines, as the development of neuroscience and neurotechnology is an international effort. Future guidelines should reflect standards that members of a society can comply with voluntarily through the process of continuous revision and supplementation in accordance with the changing environment.
Notes
References
1. Stilgoe J, Owen R, Macnaghten P. Developing a framework for responsible innovation. Res Policy. 2013; 42(9):1568–1580.
2. Owen R, Macnaghten P, Stilgoe J. Responsible research and innovation: from science in society to science for society, with society. Sci Public Policy. 2012; 39(6):751–760.
3. Salles A, Evers K, Farisco M. Neuroethics and philosophy in responsible research and innovation: the case of the Human Brain Project. Neuroethics. 2019; 12(2):201–211.
4. Lee S, Uh S, Li Z. Existing Neuroethics Guidelines. Global Neuroethics Summit 2020.
5. Zimmer A. A Neuroethics Intergration Landscape Report. Global Neuroethics Summit 2020-2021.
6. Ogbogu U, Ahmed N. Ethical, Legal, and Social Implications (ELSI) research: methods and approaches. Curr Protoc. 2022; 2(1):e354. PMID: 35041252.
7. OECD. OECD Recommendation on Responsible Innovation in Neurotechnology. Updated 2019. Accessed May 2, 2022.
https://www.oecd.org/science/recommendation-on-responsible-innovation-in-neurotechnology.htm
.
8. Nuffield Council on Bioethics. Novel Technologies: Intervening in the Brain. London, UK: Nuffield Council on Bioethics;2013.
9. Presidential Commission for the Study of Bioethical Issues. Gray Matters: Topics at the Intersection of Neuroscience, Ethics, and Society, Volume II. Updated 2015. Accessed April 20, 2022.
https://bioethicsarchive.georgetown.edu/pcsbi/sites/default/files/GrayMatter_V2_508.pdf
.
10. Greely HT, Grady C, Ramos KM, Chiong W, Eberwine J, Farahany NA, et al. Neuroethics guiding principles for the NIH BRAIN Initiative. J Neurosci. 2018; 38(50):10586–10588. PMID: 30541767.
11. Nam SM, Choi MY. Major issues and prospects of neuroethics. Focusing on the analysis of existing neuroethical guidelines. Asia Pac J Health Law Ethics. 2022; 15(3):1–30.
12. Cinel C, Valeriani D, Poli R. Neurotechnologies for human cognitive augmentation: current state of the art and future prospects. Front Hum Neurosci. 2019; 13:13. PMID: 30766483.
13. Hescham S, Liu H, Jahanshahi A, Temel Y. Deep brain stimulation and cognition: translational aspects. Neurobiol Learn Mem. 2020; 174:107283. PMID: 32739395.
14. Wu Y, Mo J, Sui L, Zhang J, Hu W, Zhang C, et al. Deep brain stimulation in treatment-resistant depression: a systematic review and meta-analysis on efficacy and safety. Front Neurosci. 2021; 15:655412. PMID: 33867929.
15. Chang CH, Chen SY, Hsiao YL, Tsai ST, Tsai HC. Hypomania with hypersexuality following bilateral anterior limb stimulation in obsessive-compulsive disorder. J Neurosurg. 2010; 112(6):1299–1300. PMID: 19911886.
16. Gilbert F, Goddard E, Viaña JNM, Carter A, Horne M. I miss being me: phenomenological effects of deep brain stimulation. AJOB Neurosci. 2017; 8(2):96–109.
17. Goering S, Brown T, Klein E. Neurotechnology ethics and relational agency. Philos Compass. 2021; 16(4):e12734. PMID: 34531923.
18. Stieglitz T. Of man and mice: translational research in neurotechnology. Neuron. 2020; 105(1):12–15. PMID: 31951526.
19. Kaebnick GE, Heitman E, Collins JP, Delborne JA, Landis WG, Sawyer K, et al. Precaution and governance of emerging technologies. Science. 2016; 354(6313):710–711. PMID: 27846595.
20. Garden H, Bowman DM, Haesler S, Winickoff DE. Neurotechnology and society: strengthening responsible innovation in brain science. Neuron. 2016; 92(3):642–646. PMID: 27810009.
21. Illes J, Blakemore C, Hansson MG, Hensch TK, Leshner A, Maestre G, et al. International perspectives on engaging the public in neuroethics. Nat Rev Neurosci. 2005; 6(12):977–982. PMID: 16340957.
22. Illes J, Moser MA, McCormick JB, Racine E, Blakeslee S, Caplan A, et al. Neurotalk: improving the communication of neuroscience research. Nat Rev Neurosci. 2010; 11(1):61–69. PMID: 19953102.
23. McCoy LG, Brenna C, Morgado F, Chen S, Das S. Neuroethics, neuroscience, and the project of human self-understanding. AJOB Neurosci. 2020; 11(3):207–209. PMID: 34029491.
24. Global Neuroethics Summit Delegates. Rommelfanger KS, Jeong SJ, Ema A, Fukushi T, Kasai K, et al. Neuroethics questions to guide ethical research in the international brain initiatives. Neuron. 2018; 100(1):19–36. PMID: 30308169.
25. Choi MY, Kim CS. Criminal Law Issues Regarding Surgeries Using Surgical Robots. Seoul, Korea: Korean Institute of Criminology and Justice;2017.
26. Kim JY. Medical Disputes and Law. 2nd ed. Seoul, Korea: Yulgok Publishing Company;2015.
27. Schneider L. Neue Behandlungsmethoden im Arzthaftungsrecht. Berlin, German: Springer;2010.
28. Schmitz-Luhn B, Katzenmeier C, Woopen C. Law and ethics of deep brain stimulation. Int J Law Psychiatry. 2012; 35(2):130–136. PMID: 22244083.
29. Desmoulin-Canselier S. Ethical and legal issues in deep brain stimulation: an overview. D’Aloia A, Errigo MC, editors. Neuroscience and Law: Complicated Crossings and New Perspectives. Cham, Switzerland: Springer;2020. p. 319–337.
30. Glannon W. Stimulating brains, altering minds. J Med Ethics. 2009; 35(5):289–292. PMID: 19407032.
31. Wexler A. A pragmatic analysis of the regulation of consumer transcranial direct current stimulation (TDCS) devices in the United States. J Law Biosci. 2015; 2(3):669–696. PMID: 27774217.
32. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices (Medical Device Regulation, MDR), Article 1, Number 2.
33. Bae JD. General Principles of Criminal Law. 15th ed. Seoul, Korea: Hongmunsa;2020.
34. Lavazza A. Free will and autonomy in the age of neurotechnologies. Lopez-Silva P, Valera L, editors. Protecting the Mind. Cham, Switzerland: Springer;2022. p. 41–58.
35. Parastarfeizabadi M, Kouzani AZ. Advances in closed-loop deep brain stimulation devices. J Neuroeng Rehabil. 2017; 14(1):79. PMID: 28800738.
36. Rainey S, Martin S, Christen A, Mégevand P, Fourneret E. Brain recording, mind-reading, and neurotechnology: ethical issues from consumer devices to brain-based speech decoding. Sci Eng Ethics. 2020; 26(4):2295–2311. PMID: 32356091.
37. Ienca M. Neuroprivacy, neurosecurity and brain-hacking: emerging issues in neural engineering. Bioethica Forum. 2015; 8(2):51–53.
38. Kreitmair KV. Dimensions of ethical direct-to-consumer neurotechnologies. AJOB Neurosci. 2019; 10(4):152–166. PMID: 31642755.
39. Lee EY. A study on the improvement of legislation regarding clinical trials. Northeast Asian Law Journal. 2020; 13(3):163–184.
40. Parens E. What does enhancement mean?. Parens E, editor. Enhancing Human Traits: Ethical and Social Implications. Washington D.C., USA: Georgetown University Press;1998.
41. Lenk C. Therapie und Enhancement: Ziele und Grenzen der modernen Medizin. Muenster, Germany: LIT Verlag;2002.
42. Buchanan A. Better Than Human: The Promise and Perils of Enhancing Ourselves. New York, NY, USA: Oxford University Press;2011.
43. Schöne-Seifert B, Talbot D, Opolka U, Ach JS. Neuro-Enhancement: Ethik vor neuen Herausforderungen. Paderborn, Germany: Mentis;2009.
44. Knoepffler N, Savulescu J. Der neue Mensche?: Enhancement und Genetik. München, Germany: Verlag Karl Alber;2009.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download