INTRODUCTION
Arthroscopic rotator cuff tear repair is associated with moderate to severe postoperative pain, and various modalities have been studied to alleviate it.
1 Interscalene brachial plexus block (ISB) provides effective analgesia in the immediate postoperative period; however, rebound pain may occur after its effect wears off.
2 A previous study has shown that compared with general anesthesia, single-shot brachial plexus block might be associated with severe rebound pain that compromises the overall benefits of regional anesthesia by decreasing the patient’s well-being and increasing the risk of unplanned healthcare resource utilization for post-discharge pain control.
3 Therefore, it is crucial to recognize and prevent rebound pain in patients who receive regional anesthesia.
Strategies to extend the analgesic duration have been suggested to reduce rebound pain.
4 As an adjuvant to ISB, dexamethasone has been proven to prolong the duration of ISB when administered either perineurally or intravenously.
5678 Our recent study demonstrated that perineural dexamethasone not only prolongs the analgesic duration of ISB, but also significantly decreases the incidence of rebound pain after its effect wears off.
9 While perineural dexamethasone has been found to be equivalent or even superior to intravenous dexamethasone in terms of prolonging the block duration, routine use of perineural dexamethasone is limited by the fact that it represents off-label use and its long-term safety remains inconclusive. These limitations have promoted the use of intravenous dexamethasone, which is also known to decrease postoperative pain and prevent postoperative nausea and vomiting as a component of multimodal analgesia.
1011
To date, most studies comparing the effects of perineural and intravenous dexamethasone have focused on analgesic duration and not specifically on the occurrence of rebound pain after ISB resolution. In the current study, we hypothesized that perineural and intravenous dexamethasone would affect rebound pain differently in patients who received ISB for arthroscopic rotator cuff tear repair.
METHODS
Study design and participants
Patients aged ≥ 20 years with an American Society of Anesthesiologists physical status of 1–3, who were scheduled for elective arthroscopic rotator cuff tear repair under general anesthesia with preoperative single-injection ISB between December 2021 and March 2022, were enrolled. Patients with coagulation disorders, neuropathic disorders, diaphragmatic paralysis, allergies to local anesthetics, severe cardiopulmonary disease, systemic steroid use, uncontrolled diabetes, chronic opioid use, psychiatric disorders, previous ipsilateral shoulder surgery, and inability to understand pain scoring or instructions for intravenous patient-controlled analgesia (PCA) were excluded.
Randomization
The patients were randomly assigned to either the perineural dexamethasone group (perineural group) or the intravenous dexamethasone group (intravenous group). In the perineural group, patients received single-injection ISB with 12 mL of 0.5% ropivacaine (60 mg) containing 5 mg of dexamethasone; simultaneously, 1 mL of 0.9% normal saline was administered intravenously. In the intravenous group, patients received single-injection ISB with 12 mL of 0.5% ropivacaine (60 mg); simultaneously, 1 mL of dexamethasone (5 mg) was administered intravenously. Prior to the study, a researcher who did not participate in the study applied a random allocation sequence at a ratio of 1:1 using a computer-generated randomization table. Sealed opaque envelopes were used to conceal the allocations. A research assistant prepared the intervention drug according to the randomization and delivered it to the attending anesthesiologists. All the intervention drugs were identical clear-colored, indistinguishable for drug composition. The patients, surgeons, attending anesthesiologists, and outcome assessors were unaware of the group allocations.
Anesthesia
All patients were administered ultrasound-guided single-injection ISB by one of two experienced anesthesiologists (HJL or JHW) as described previously.
9 Midazolam (3 mg) and fentanyl (50 μg) were used for sedation prior to ISB. A high-frequency linear array transducer (13–6 MHz) was placed over the interscalene region to identify the brachial plexus in a short-axis view. A 50 mm, 22-gauge facet tip needle was advanced aseptically using the in-plane method until the needle tip was adjacent to the C-5 and C-6 spinal nerve roots. After confirming negative aspiration, 12 mL of the perineural study drug was administered and allowed to spread around the C-5 and C-6 spinal nerve roots. Simultaneously, 1 mL of intravenous study drug solution was also injected intravenously.
General anesthesia was induced using glycopyrrolate (0.2 mg), propofol (2 mg.kg
−1), fentanyl (1–2 μg.kg
−1), and rocuronium (0.6 mg.kg
−1), followed by tracheal intubation. Anesthesia was maintained using 50% oxygen in air and desflurane, with a bispectral index of 40–60. A bolus of fentanyl and/or nicardipine was administered as needed to maintain the systolic blood pressure at < 100 mmHg upon the surgeon’s request. The maximum dose of fentanyl used during induction or during surgery was within the limit of 2 μg.kg
−1. Unlike in our previous study,
9 continuous infusion of remifentanil was not used in this study because intraoperative remifentanil infusion reportedly induces postoperative hyperalgesia.
1213 A single surgeon performed all the surgeries. At the end of the surgery, 0.3 mg of ramosetron was administered to prevent nausea. Tracheal extubation was performed in the operating room after reversing residual muscle relaxation.
Pre- and postoperative pain management
All patients were scheduled to be admitted on the day before surgery and discharged on postoperative day (POD) 2. According to our standardized protocol, celecoxib (200 mg) and pregabalin (25 mg) were administered in the evening of the day before surgery. After surgery, pain intensity was measured using the numeric rating scale (NRS), ranging from 0 to 10, with 0 being “no pain” and 10 being “the worst imaginable”, and loss of cold sensation in the C-5 and C-6 sensory dermatomes was assessed in the recovery room. ISB was established under ultrasound guidance, which improved its success rate; therefore, block failure was not checked for before general anesthesia induction in the operation room, thereby avoiding delay of surgery. Block failure was confirmed when the patients rated surgical pain with an NRS score > 3 and did not experience a loss of cold sensation in the C-5 and C-6 sensory dermatomes in the recovery room.
9 Intravenous PCA device (Accumate
® 1200; Woo Young Meditech, Seoul, Korea) was initiated on arrival in the recovery room, and was set to deliver 1 mL.hour
−1 with a 1 mL bolus dose (0.9% normal saline and 10 μg.mL
−1 fentanyl, lockout time of 15 minutes). Patients were instructed to press the button for PCA bolus if they started to feel that the ISB was not providing sufficient pain relief.
In the ward, the patients were given buprenorphine patches (5 μg.hr-1) on the day of surgery. After the “nil per os” time, the patients took tramadol (37.5 mg)-acetaminophen (325 mg) combination tablets orally every 12 hours. An additional 30 mg of ketorolac (up to 60 mg.day−1) or 100 mg of tramadol (up to 300 mg.day−1) was administered if pain control was insufficient. Data regarding the use of PCA devices were collected using AccuLinker software (data extraction program of Accumate® 1200 version 1.1; Woo Young Meditech). After discharge, the patients continued using buprenorphine patches and the tramadol (37.5 mg)-acetaminophen (325 mg) combination tablets for 2 weeks.
Outcome assessment
The primary outcome was the difference in the pain score between before and after ISB resolution. To calculate the increase in pain after ISB resolution in all patients, the lowest pain score during the first 12 hours before the ISB had worn off was subtracted from the highest pain score during the first 12 hours after the ISB had worn off.
29 All of pain scores were assessed when the patient was at rest, as our rehabilitation protocol is to immobilize the operated shoulder until the initiation of passive range-of-motion exercises one month postoperatively. The secondary outcomes were the incidence of rebound pain; time to the first analgesic request; onset, duration, and intensity of rebound pain; occurrence of sleep disturbances due to surgical-site pain; pain score at predefined time points; and PCA fentanyl and rescue analgesic consumption during the first 48 hours after surgery. Rebound pain was defined as severe pain (NRS score ≥ 7) at the surgical site after ISB resolution. The time to the first analgesic request, defined as the interval between ISB and the first analgesic administration, was regarded as the analgesic duration. If a patient did not request for postoperative analgesics during hospitalization, the analgesic duration was defined as the time until the patient felt a recovery of sensation on the corresponding dermatomes. The onset, duration, and intensity of rebound pain and occurrence of sleep disturbances due to surgical-site pain were recorded. The onset of rebound pain was recorded in two ways, namely from the time of performing ISB and from the time of ISB resolution to the occurrence of rebound pain. The patients were asked to complete a pain diary when they felt that the ISB did not inhibit their surgical pain and the pain suddenly increased. A research assistant visited patients at 12, 24, and 48 hours postoperatively to check their pain score, a pain diary, and whether additional analgesic was administered at the time of visit and between visits. Complications, including postoperative nausea and vomiting, were also recorded. Patients were contacted on POD 7 by phone call to determine whether they had experienced severe pain, sleep disturbances due to surgical-site pain, or any adverse effects after hospital discharge.
Statistical analysis
The SPSS (ver. 26.0; IBM Corp., Armonk, NY, USA) was used for statistical analysis. From our prior experience and the previous study,
9 we expected the difference in pain score before and after ISB resolution of 4.6 ± 2.0, and a 30% difference in that value between two groups would be clinically significant (two-tailed
t-test: α = 0.05, power = 80%). Considering a dropout rate of 10%, 38 patients were required per group. Continuous data were analyzed using an independent
t-test or the Mann-Whitney
U test according to the normality of the data. Categorical variables were analyzed using the χ
2 test. Statistical significance was set at
P < 0.05. The data are presented as mean ± standard deviation, median (interquartile range; IQR), or number (percentage) as appropriate.
Ethics statement
This study was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Ewha Womans University, Seoul, Republic of Korea on November 23, 2021 (approval No.: SEUMC 2019-09-036-001). This study was registered in the Clinical Trial Registry of Korea (No.
KCT0006795, December 2, 2021, cris.nih.go.kr). The first patient was enrolled on December 14, 2021, and written informed consent was obtained from all the patients.
RESULTS
The flow diagram of the Consolidated Standards of Reporting Trials is shown in
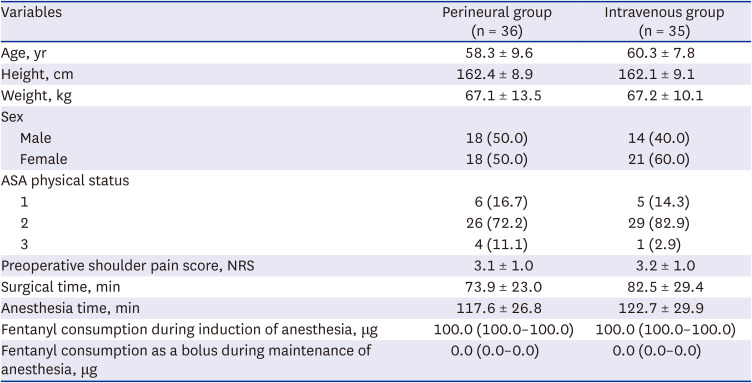
Fig. 1. Seventy-nine patients were assessed for eligibility. Among them, two patients were excluded because of a history of ipsilateral shoulder surgery, and one patient due to refusal to participate. Thus, 76 patients were allocated to either the perineural or the intravenous group. After allocation, the five patients were excluded from the final analysis. In the perineural group, one patient was excluded for facing difficulties in communicating and expressing pain because delirium occurred immediately after surgery and another withdrew consent to participate in the study after the surgical procedure. In the intravenous group, one patient was excluded because steroids had been administered to treat bronchospasms during anesthesia emergence, a second one underwent simple synovectomy instead of rotator cuff repair as per the intraoperative decision, and a third one withdrew consent to participate in the study after surgery. Overall, 71 patients were included for final analysis. ISB was performed successfully in all patients. The patient characteristics and intraoperative clinical data were similar between the two groups (
Table 1).
Fig. 1
Flow diagram of patient recruitment.
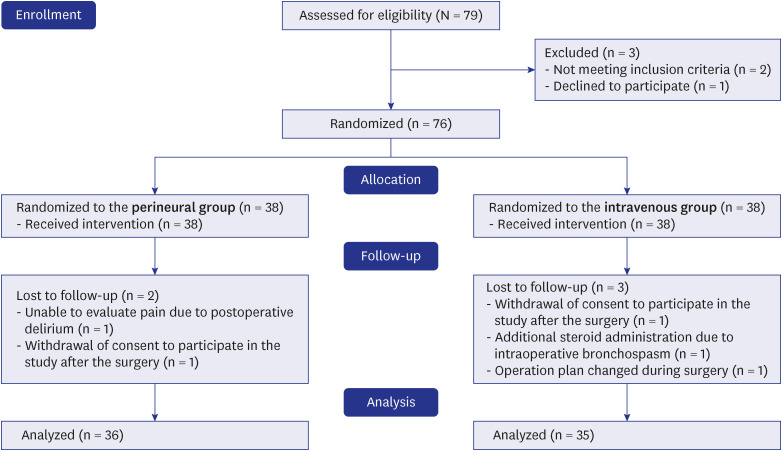
Table 1
Characteristics and intraoperative data of the perineural and intravenous group
|
Variables |
Perineural group (n = 36) |
Intravenous group (n = 35) |
|
Age, yr |
58.3 ± 9.6 |
60.3 ± 7.8 |
|
Height, cm |
162.4 ± 8.9 |
162.1 ± 9.1 |
|
Weight, kg |
67.1 ± 13.5 |
67.2 ± 10.1 |
|
Sex |
|
|
|
Male |
18 (50.0) |
14 (40.0) |
|
Female |
18 (50.0) |
21 (60.0) |
|
ASA physical status |
|
|
|
1 |
6 (16.7) |
5 (14.3) |
|
2 |
26 (72.2) |
29 (82.9) |
|
3 |
4 (11.1) |
1 (2.9) |
|
Preoperative shoulder pain score, NRS |
3.1 ± 1.0 |
3.2 ± 1.0 |
|
Surgical time, min |
73.9 ± 23.0 |
82.5 ± 29.4 |
|
Anesthesia time, min |
117.6 ± 26.8 |
122.7 ± 29.9 |
|
Fentanyl consumption during induction of anesthesia, μg |
100.0 (100.0–100.0) |
100.0 (100.0–100.0) |
|
Fentanyl consumption as a bolus during maintenance of anesthesia, μg |
0.0 (0.0–0.0) |
0.0 (0.0–0.0) |
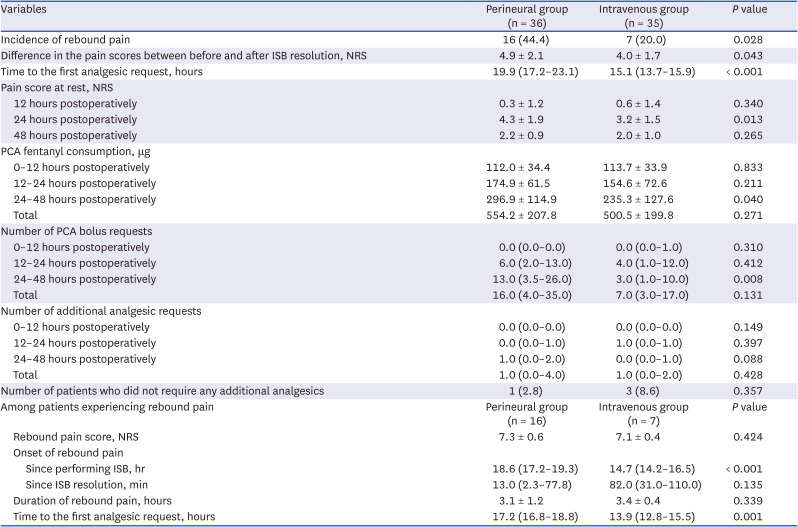
The difference in the pain scores between before and after ISB resolution was greater in the perineural group compared to the intravenous group (4.9 ± 2.1 vs. 4.0 ± 1.7,
P = 0.043;
Table 2). The incidence of rebound pain was significantly higher in the perineural group compared to the intravenous group (44.4% vs. 20.0%,
P = 0.028). The time to the first analgesic request was more prolonged in the perineural group (19.9 [17.2–23.1] hours) compared to the intravenous group (15.1 [13.7–15.9] hours,
P < 0.001), with 3.7-hour difference by the equal variance t-test. The pain scores at 24 hours after surgery were lower in the intravenous group (
P = 0.013), and the amount of PCA fentanyl consumption was greater in the perineural group between 24 and 48 hours (
P = 0.040). Pain scores assessed at other time points did not differ significantly between the two groups.
Table 2
Rebound pain and postoperative data of the perineural and intravenous groups.
|
Variables |
Perineural group (n = 36) |
Intravenous group (n = 35) |
P value |
|
Incidence of rebound pain |
16 (44.4) |
7 (20.0) |
0.028 |
|
Difference in the pain scores between before and after ISB resolution, NRS |
4.9 ± 2.1 |
4.0 ± 1.7 |
0.043 |
|
Time to the first analgesic request, hours |
19.9 (17.2–23.1) |
15.1 (13.7–15.9) |
< 0.001 |
|
Pain score at rest, NRS |
|
|
|
|
12 hours postoperatively |
0.3 ± 1.2 |
0.6 ± 1.4 |
0.340 |
|
24 hours postoperatively |
4.3 ± 1.9 |
3.2 ± 1.5 |
0.013 |
|
48 hours postoperatively |
2.2 ± 0.9 |
2.0 ± 1.0 |
0.265 |
|
PCA fentanyl consumption, μg |
|
|
|
|
0–12 hours postoperatively |
112.0 ± 34.4 |
113.7 ± 33.9 |
0.833 |
|
12–24 hours postoperatively |
174.9 ± 61.5 |
154.6 ± 72.6 |
0.211 |
|
24–48 hours postoperatively |
296.9 ± 114.9 |
235.3 ± 127.6 |
0.040 |
|
Total |
554.2 ± 207.8 |
500.5 ± 199.8 |
0.271 |
|
Number of PCA bolus requests |
|
|
|
|
0–12 hours postoperatively |
0.0 (0.0–0.0) |
0.0 (0.0–1.0) |
0.310 |
|
12–24 hours postoperatively |
6.0 (2.0–13.0) |
4.0 (1.0–12.0) |
0.412 |
|
24–48 hours postoperatively |
13.0 (3.5–26.0) |
3.0 (1.0–10.0) |
0.008 |
|
Total |
16.0 (4.0–35.0) |
7.0 (3.0–17.0) |
0.131 |
|
Number of additional analgesic requests |
|
|
|
|
0–12 hours postoperatively |
0.0 (0.0–0.0) |
0.0 (0.0–0.0) |
0.149 |
|
12–24 hours postoperatively |
0.0 (0.0–1.0) |
1.0 (0.0–1.0) |
0.397 |
|
24–48 hours postoperatively |
1.0 (0.0–2.0) |
0.0 (0.0–1.0) |
0.088 |
|
Total |
1.0 (0.0–4.0) |
1.0 (0.0–2.0) |
0.428 |
|
Number of patients who did not require any additional analgesics |
1 (2.8) |
3 (8.6) |
0.357 |
|
Among patients experiencing rebound pain |
Perineural group (n = 16) |
Intravenous group (n = 7) |
P value |
|
Rebound pain score, NRS |
7.3 ± 0.6 |
7.1 ± 0.4 |
0.424 |
|
Onset of rebound pain |
|
|
|
|
|
Since performing ISB, hr |
18.6 (17.2–19.3) |
14.7 (14.2–16.5) |
< 0.001 |
|
|
Since ISB resolution, min |
13.0 (2.3–77.8) |
82.0 (31.0–110.0) |
0.135 |
|
Duration of rebound pain, hours |
3.1 ± 1.2 |
3.4 ± 0.4 |
0.339 |
|
Time to the first analgesic request, hours |
17.2 (16.8–18.8) |
13.9 (12.8–15.5) |
0.001 |
Among the patients who experienced rebound pain, the rebound pain scores were similar between the perineural and intravenous groups (7.3 ± 0.6 vs. 7.1 ± 0.4;
Table 2). The onset of rebound pain after ISB was performed was more prolonged in the perineural group (18.6 [17.2–19.3] hours) compared to the intravenous group (14.7 [14.2–16.5] hours,
P < 0.001). There was no significant difference in the timing of the occurrence of rebound pain after ISB resolution between the perineural and intravenous groups (13 [2.3–77.8] and 82 [31.0–110.0] minutes, respectively). Rebound pain lasted for approximately 3 hours in both groups.
As shown in
Fig. 2, the incidence of sleep disturbance due to surgical-site pain was higher in the perineural group than in the intravenous group on the night of the surgery (50.0% vs. 25.7%,
P = 0.035) and during the first postoperative week (55.6% vs. 25.7%,
P = 0.011). The rates of postoperative nausea and vomiting were similar between the groups. All patients were discharged from the hospital on the morning of POD 2 as scheduled. Up to POD 7, none of the patients had experienced complications such as persistent paresthesia or motor weakness in the upper arm (
Table 3).
Fig. 2
Patients experiencing sleep disturbances due to pain at the surgical-site during the first postoperative week. Data are presented as proportions, with lines representing the 95% confidence interval for the proportion estimated using the Clopper-Pearson method. Statistically significant differences between the two groups, as determined by the x2 test, were observed during the first postoperative week (P = 0.011) and on the night of the surgery (P = 0.035). Perineural group (blue bar); intravenous group (red bar).
POD = postoperative day.
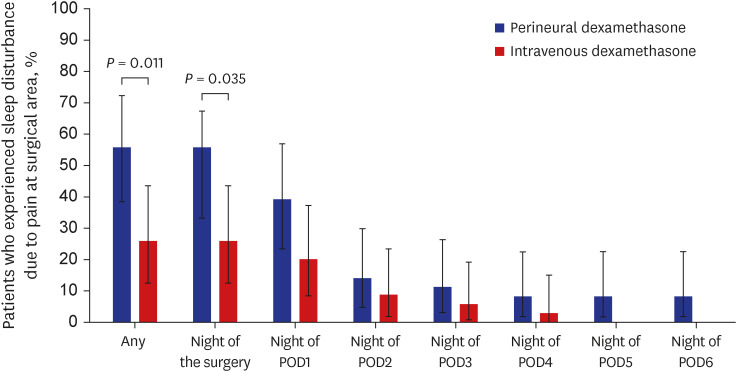
Table 3
Block characteristics and adverse effects in the perineural and intravenous groups
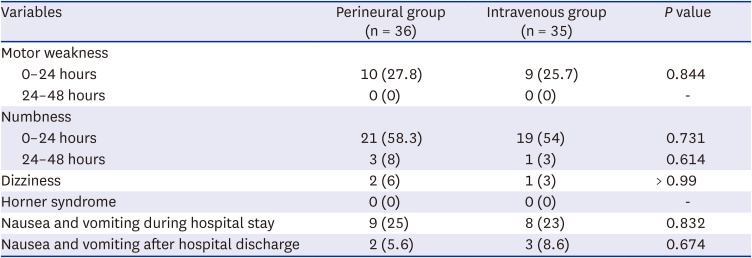
|
Variables |
Perineural group (n = 36) |
Intravenous group (n = 35) |
P value |
|
Motor weakness |
|
|
|
|
0–24 hours |
10 (27.8) |
9 (25.7) |
0.844 |
|
24–48 hours |
0 (0) |
0 (0) |
- |
|
Numbness |
|
|
|
|
0–24 hours |
21 (58.3) |
19 (54) |
0.731 |
|
24–48 hours |
3 (8) |
1 (3) |
0.614 |
|
Dizziness |
2 (6) |
1 (3) |
> 0.99 |
|
Horner syndrome |
0 (0) |
0 (0) |
- |
|
Nausea and vomiting during hospital stay |
9 (25) |
8 (23) |
0.832 |
|
Nausea and vomiting after hospital discharge |
2 (5.6) |
3 (8.6) |
0.674 |
DISCUSSION
We demonstrated that compared with the use of perineural dexamethasone, the use of intravenous dexamethasone as an adjuvant to ISB using ropivacaine reduced the overall extent of pain increase after ISB resolution and the incidence of rebound pain (NRS ≥ 7) significantly from 44.4% to 20.0%. Although the time to the first analgesic duration was several hours longer in the perineural group than in the intravenous group, the more beneficial effect on rebound pain in the intravenous group was considered to contribute to the lower pain scores at 24 hours and lower opioid consumption during the postoperative 24–48 hours. The number of patients experiencing sleep disturbance due to surgical-site pain on the night of the surgery and during the first postoperative week in the intravenous group was approximately half of that in the perineural group, with a significant difference between the groups. Among the patients who experienced rebound pain, the mean NRS scores and durations of rebound pain were similar between the two groups, regardless of the route of dexamethasone administration.
Addition of dexamethasone to ISB is a simple well-known strategy for prolonging its analgesic duration. Previous studies have shown that compared with intravenous administration, perineural administration of dexamethasone for ISB prolonged the duration of analgesia by ≤ 5 hours.
141516 This is consistent with our result of a 3.7-hour prolongation (difference in means) in the perineural group compared with the duration in the intravenous group. Given the relatively small difference of analgesic prolongation and the off-label use of perineural dexamethasone, some authors might debate whether perineural dexamethasone should be used routinely or be reserved for selected patients for whom this prolongation would be clinically beneficial.
16 Of note, our results showed a significant reduction in the incidence of rebound pain with the use of intravenous dexamethasone, and this might serve as a meaningful basis for clinical judgment. In the current study, rebound pain was observed at 18.6 hours and 14.7 hours from the time of performing ISB in the perineural and intravenous groups, respectively. However, the incidence of rebound pain in the intravenous group was approximately half of that in the perineural group.
Considering the typical pain trajectory after ISB for arthroscopic shoulder surgery, patients experience minimal or no pain during the analgesic duration of ISB.
49 Once the ISB wears off, the patient is exposed to pre-existing nociceptive stimuli, mainly primary hyperalgesia at the surgical site and secondary hyperalgesia in the surrounding uninjured tissues, that are triggered by surgical tissue injury and lasting for up to several days after surgery.
4 Severe pain at this time is referred to as rebound pain; it is the most intense pain that patients experience in their postoperative course after receiving regional anesthesia. Therefore, reducing the incidence and intensity of rebound pain is a key strategy for successful regional anesthesia as well as for prolonging the analgesic duration. While the analgesic effect lasts for a shorter duration with intravenous dexamethasone than with perineural dexamethasone, the significantly lower incidence of rebound pain with intravenous dexamethasone compensates for this limitation, allowing for a smooth transition from ISB. In our previous study,
9 compared with ISB without dexamethasone, perineural dexamethasone reduced rebound pain after ISB. In this study, compared with perineural dexamethasone, intravenous dexamethasone reduced the incidence of rebound pain further.
Rebound pain could reportedly be reduced by prolonging the analgesic duration.
4 However, our results imply that a prolonged analgesic duration is not necessarily associated with a reduced incidence of rebound pain. Given that the typical inflammatory response after surgery lasts for several days, the commonly used techniques to prolong the effect of ISB (e.g., continuous catheter, adjuvant to single nerve block) fall short in overcoming nociceptive pain. Therefore, reducing the inflammatory response could be a reasonable measure, although the mechanism of rebound pain remains unclear. We hypothesized that systemic dexamethasone partly plays a role in reducing rebound pain efficiently through systemic anti-inflammatory effects.
It is unclear why intravenous dexamethasone performed better in reducing rebound pain. First, the reason and mechanism of differences in dexamethasone activity between the perineural and intravenous routes remain unknown. When administered perineurally, dexamethasone causes vasoconstriction to decrease the absorption of local analgesics or inhibit potassium channel-mediated discharge of nociceptive C-fibers, thereby increasing the duration of the peripheral nerve block.
17 It has also been suggested to have systemic anti-inflammatory effects.
18 It is unclear whether intravenous dexamethasone works only systemically or has local effects as well, and whether using the same amount of dexamethasone via different routes is suitable for comparing their efficacy. As equivalent doses for different routes of dexamethasone administration have not been established, we assessed the incidence of rebound pain with a commonly prescribed dose of dexamethasone administered via different routes. One meta-analysis suggested that a higher concentration of perineural dexamethasone than that of intravenous dexamethasone might be required to achieve analgesic efficacy.
19 However, there is no rationale for using off-label perineural dexamethasone if a higher concentration is needed to produce a systemic effect equivalent to that of intravenous dexamethasone. Lastly, some authors have described the risk of exacerbated local anesthetic-induced hyperalgesia following co-administration of perineural dexamethasone.
20 They have suggested that intravenous dexamethasone had an analgesic effect, whereas a high concentration of perineural dexamethasone (≥ 8 mg) could induce hyperalgesia; this should be clarified in future studies.
While there are several proposed definitions for rebound pain, the unifying characteristic is acute postoperative pain that occurs after the effect of peripheral nerve block resolves, which is clinically significant. Identifying “clinically significant” pain is challenging for researchers, since it is inherently subjective. Therefore, we considered that the patient experienced rebound pain when the NRS score was ≥ 7 at the surgical site after ISB resolution. There have been many studies on dexamethasone administration for ISB. Most studies focused on the time until the block wore off by checking the pain score at a predefined time point, but this method may miss or underestimate the transient presence of rebound pain. The strength of our study is that we specifically focused on identifying rebound pain. While arthroscopic shoulder surgery is often performed on an ambulatory basis, patients are routinely discharged on the morning of POD 2 in our center, which allows us to keep track of their response. In addition to regular visits by assessors, patients were instructed to complete a pain diary when they felt that the ISB did not inhibit their surgical pain or they felt a sudden increase in pain, and to press a call button to the nursing station when pain control was insufficient despite the use of PCA.
Notably, the intensity and duration of rebound pain were similar between the two routes of dexamethasone administration, despite a significant reduction in the incidence of rebound pain with intravenous dexamethasone. In our previous study, a similar duration of rebound pain (approximately 3 hours) was found in patients who received ISB without dexamethasone.
9 These results provide a basis for future studies to investigate measures to reduce the duration and severity of rebound pain.
This study had some limitations. First, this was a single-center study with a relatively small sample size and was not powered to detect differences in the adverse effects between the two routes. Second, block duration was defined as the time to the first analgesic request or the time taken before the patients recovered their sensation in cases where they did not request for analgesics. However, the ISB could wear off during sleep; hence, patients might request for analgesics when they wake up from their sleep on realizing that the block has worn off. Third, our results cannot be applied to regional anesthesia other than ISB because the effects of an adjuvant to local anesthetics might vary with different nerve blocks.
18 In addition, these results cannot be generalized to surgeries other than arthroscopic rotator cuff tear repair, which might follow different healing processes and consequent pain trajectories.
In conclusion, intravenous dexamethasone as an adjuvant to ISB reduced the increase in pain after ISB resolution, the incidence of rebound pain, and the incidence of pain-related sleep disturbance in the first postoperative week, although the duration of analgesia was several hours shorter with intravenous dexamethasone than with perineural dexamethasone. Hence, as an adjuvant to ISB, intravenous dexamethasone might allow a smoother transition to postoperative analgesia compared with the off-label use of perineural dexamethasone in patients undergoing arthroscopic rotator cuff tear repair.








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download