This article has been
cited by other articles in ScienceCentral.
PRESENTATION OF CASE 10
Dr. Kabsoo Shin: A 30-year-old female visited the clinic for evaluation of a palpable mass in her right breast. She had no specific past medical history, and the mass was detected two weeks prior to her visit. She also complained of intermittent oozing eczema on her left nipple for several years.
On physical examination, a hard and irregular mass was palpable in the upper quadrant of her right breast, and the mass was about 3 cm in size. Right axillary lymph node was also palpable. There was eczematous change on her left nipple.
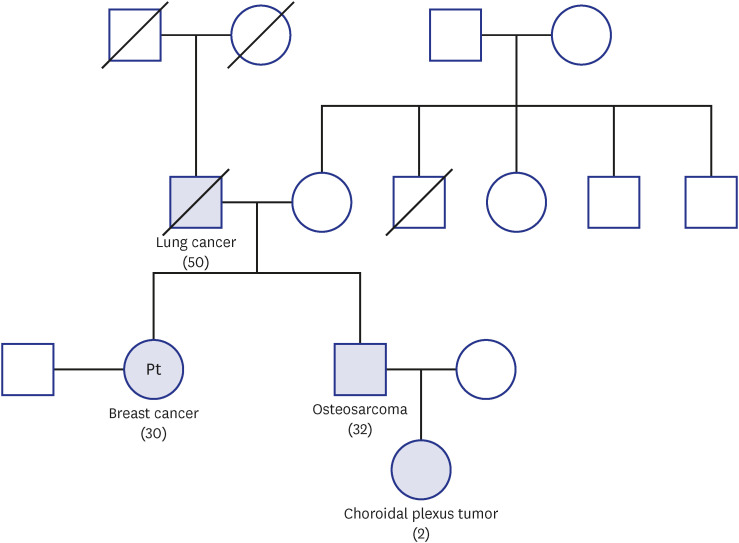
The patient’s menstrual cycle was regular, with a 28-day period. She had no history of taking contraceptive pills. The patient’s father passed away early due to progression of lung cancer, and her older brother was diagnosed with osteosarcoma and received systemic chemotherapy and surgery. The patient’s nephew was diagnosed with choroidal plexus tumor. The family pedigree is described in
Fig. 1.
Fig. 1
The pedigree of the patient’s family. The numbers in parentheses represent the age of onset of the disease.
There were no specific findings in the complete blood count and blood chemistry.
CLINICAL IMPRESSION
Dr. Kabsoo Shin: Breast cancer, warranting genetic study for evaluation of hereditary breast cancer.
IMAGE PRESENTATION
Dr. Kabsoo Shin: We performed breast mammography, sonography and breast magnetic resonance imaging (MRI) for initial evaluation. May we review the imaging findings?
Dr. Jeongmin Lee: Mammography shows irregular indistinct hyperdense mass at upper central region of right breast. Also, multifocal grouped amorphous microcalcifications showing segmental distribution is noted at upper outer quadrant of right breast, which is suspected to be high suspicion for malignancy (Breast Imaging Reporting and Data System [BI-RADS] category 4C) (
Fig. 2A). In addition, grouped amorphous microcalcifications is noted at lower central region of left breast, which is suspected to be low suspicion for malignancy (BI-RADS category 4A) (
Fig. 2B).
Fig. 2
(A) Mammography shows indistinct hyperdense mass upper central region of right breast (arrow) and segmental distributed multifocal grouped amorphous microcalcifications at upper outer quadrant of right breast (arrowheads). (B) Another grouped amorphous microcalcifications is noted at lower central region of left breast (arrows). (C) Indistinct hypoechoic malignant mass at 12 o’clock of right breast (arrow). Segmental ductal changes with hypoechoic nodularities (arrowheads). (D) Small but prominent lymph nodes with cortical thickening are detected in right axillary level I, which are suspicious for metastatic lymph nodes. (E) Dynamic contrast enhanced breast MRI. MRI shows irregular shaped heterogeneously enhancing malignant mass at 12 o’ clock of right breast (arrow) on early dynamic phase. Along with this mass, segmental distributed clumped NME with segmental distribution is noted at upper outer quadrant of right breast (arrowheads). (F) Multifocal clumped or clustered ring pattern NME (hollow arrows) is noted at outer region of left breast. This finding is suspected to be bilateral malignancy, which is correlated with grouped microcalcifications at left breast in mammography.
MRI = magnetic resonance imaging, NME = non-mass enhancement.
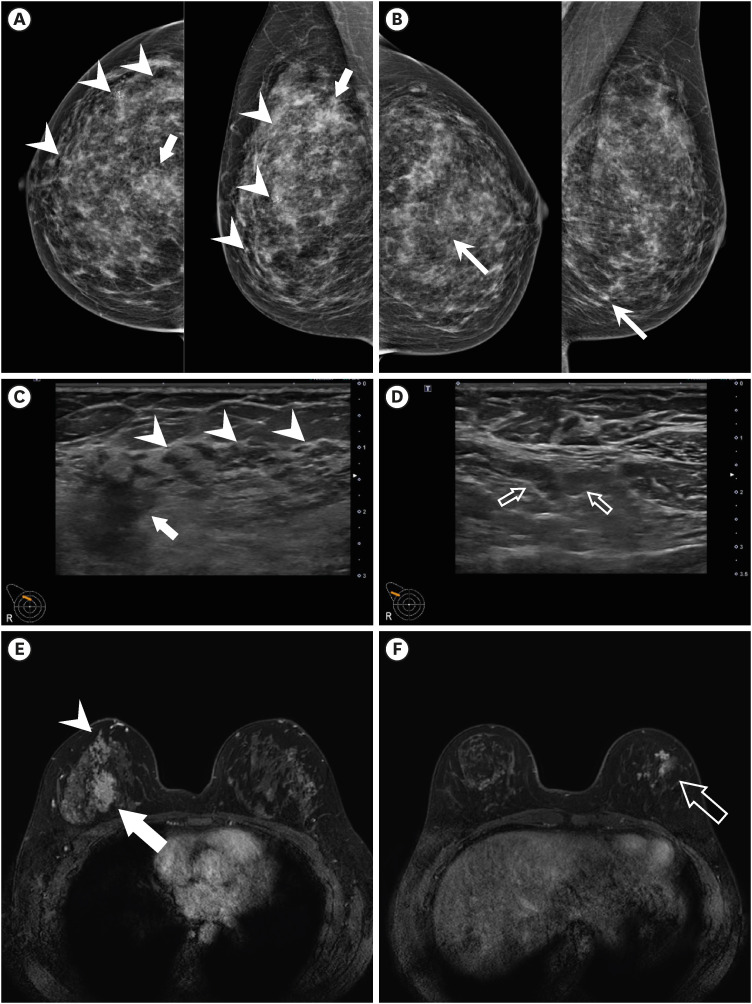
Ultrasound shows a 3 cm sized indistinct hypoechoic mass at 12 o’clock direction of right breast which is correlated with suspected malignancy by mammography (
Fig. 2C). Ill-defined hypoechoic lesions as ductal changes with nodules are also noted at upper outer quadrant of right breast which are correlated with suspicious microcalcifications in mammography (
Fig. 2C). In addition to breast lesions, three, less than 1 cm sized lymph nodes showing eccentric cortical thickening are noted at right axillary level I which are suspected as metastatic lymph nodes (
Fig. 2D). There is no suspicious finding suspected for malignancy at left breast in ultrasound.
Dynamic contrast enhanced breast MRI shows irregular shaped malignant mass with internal heterogenous enhancement at 12 o’clock of right breast (
Fig. 2E). Segmental clumped non-mass enhancement (NME) is noted at upper outer quadrant of right breast, lower outer aspect to malignant mass which is correlated with microcalcifications in mammography and ill-defined hypoechoic lesions in ultrasound (
Fig. 2E). In addition to right breast findings, multifocal clumped NME is noted in outer region of left breast, which is suspected as bilateral malignancy (
Fig. 2F). In right axillary level I, two lymph nodes with suspicious for metastases are noted.
PATHOLOGICAL FINDINGS
Dr. Jeongmin Lee: An ultrasound-guided biopsy was performed in the mass at 12 o’clock direction of the right breast at an outside hospital. The multiple NME lesions in the left breast were unable to be targeted with ultrasound. A punch biopsy of the left nipple’s skin was also performed.
Dr. Jun Kang: In the core needle biopsy specimen, invasive breast carcinoma of no special type, grade 2 was identified. It was negative for estrogen receptor (ER) and progesterone receptor (PR) expression, and positive for HER2 expression was (3+). The pathology image of the hematoxylin and eosin slide of the core needle biopsy cannot be presented here because the slide was returned to the hospital where it was previously biopsied.
The punch biopsy of the left nipple showed single cells or clusters of cells scattered throughout the epidermis. The cells had pleomorphic nuclei and abundant pale cytoplasm, consistent with Paget cells.
DIFFERENTIAL DIAGNOSIS
Dr. Kabsoo Shin: The patient was diagnosed with early-onset breast cancer and a synchronous bilateral malignancy was suspected. Her brother and nephew had osteosarcoma and choroidal plexus tumor, respectively. Her father also had history of lung cancer. What kind of hereditary condition is suspected based on her history?
Dr. Jieun Lee: Both early onset and multiple primaries of breast cancer are risk factors for a pathogenic BRCA1/2 mutation. Thus, germline BRCA1/2 testing is necessary. Breast cancer, osteosarcoma and choroidal plexus tumor are considered as Li-Fraumeni syndrome (LFS)-related tumors which are associated with germline
TP53 mutations. According to the Chompret criteria (as shown in the
Table 1), LFS is suspected, and germline
TP53 testing is needed.
12
Table 1
Classic LFS criteria and Chompret criteria12
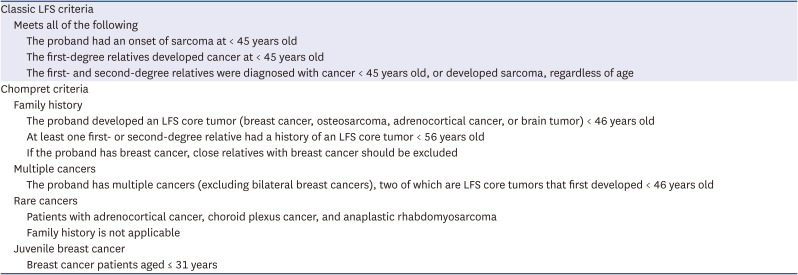
|
Classic LFS criteria |
|
Meets all of the following |
|
|
The proband had an onset of sarcoma at < 45 years old |
|
|
The first-degree relatives developed cancer at < 45 years old |
|
|
The first- and second-degree relatives were diagnosed with cancer < 45 years old, or developed sarcoma, regardless of age |
|
Chompret criteria |
|
Family history |
|
|
The proband developed an LFS core tumor (breast cancer, osteosarcoma, adrenocortical cancer, or brain tumor) < 46 years old |
|
|
At least one first- or second-degree relative had a history of an LFS core tumor < 56 years old |
|
|
If the proband has breast cancer, close relatives with breast cancer should be excluded |
|
Multiple cancers |
|
|
The proband has multiple cancers (excluding bilateral breast cancers), two of which are LFS core tumors that first developed < 46 years old |
|
Rare cancers |
|
|
Patients with adrenocortical cancer, choroid plexus cancer, and anaplastic rhabdomyosarcoma |
|
|
Family history is not applicable |
|
Juvenile breast cancer |
|
|
Breast cancer patients aged ≤ 31 years |
GERMLINE TEST FINDINGS
Dr. Jieun Lee: Germline BRCA1/2 mutations were tested with her peripheral blood by direct Sanger sequencing. Since BRCA1/2 mutations were not detected, a subsequent multigene germline test for inherited mutations was performed with peripheral blood. She was heterozygous for a pathogenic mutation, pArg248Gln(c.743G>A) in the TP53 gene.
DIAGNOSIS
Dr. Kabsoo Shin: Hormone receptor-negative, HER2-positive breast cancer in the right (cT3N1) and ductal carcinoma in situ with Paget disease in the left breast (cTisN0). A diagnosis of LFS was confirmed by the detection of a heterozygous pathogenic mutation, pArg248Gln(c.743G>A) in the TP53 gene.
CLINICAL COURSE
Dr. Kabsoo Shin: She received six cycles of neoadjuvant docetaxel, carboplatin, trastuzumab and pertuzumab (TCHP). During the neoadjuvant period, a diagnosis of LFS was confirmed. After neoadjuvant treatment, a radiologic complete response was obtained. Following genetic counseling and multidisciplinary discussion, bilateral mastectomy was planned to avoid adjuvant radiation. She underwent a right mastectomy with sentinel lymph node biopsy (SLNB) (ypT0N0) and a contralateral mastectomy with SLNB (ypTisN0). DCIS in left breast was ER- and PR-positive and HER2-negative.
As an adjuvant treatment, she was treated with twelve cycles of the combination of trastuzumab and pertuzumab and daily tamoxifen. After thirteen months of the surgery, a routine surveillance brain MRI found a 1.1 cm-sized metastatic nodule in the right cerebellum. Since there was no evidence of recurrence at other sites and the nodule was in a deep-seated location, CyberKnife radiosurgery was performed at the right cerebellum, the site of recurrent.
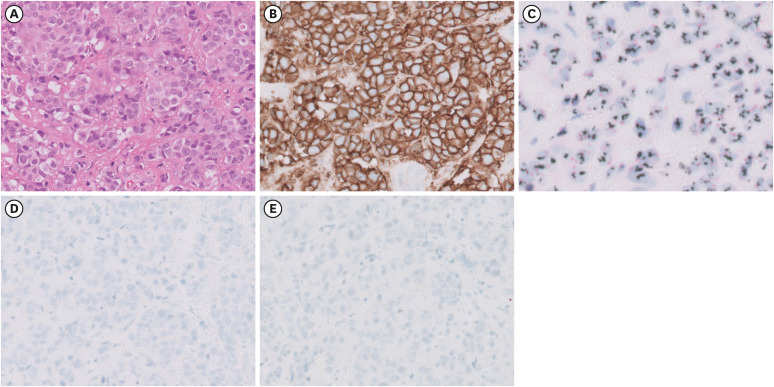
The recurrent cerebral nodule was stable for 18 months after CyberKnife radiosurgery, but regrowth was detected. After the risk and benefit assessment in a multidisciplinary discussion, a craniotomy with tumor removal was performed, followed by whole brain radiation with hippocampal sparing despite the risk of radiation-induced sequelae. In brain metastasized tissue, the tumor cells showed a lack of tubule or gland formation, moderate nuclear pleomorphism, and frequent mitosis (
Fig. 3A). The tumor cells were homogeneous, dark circumferential pattern in ERBB2 (HER2) immunostaining (
Fig. 3B). ERBB2 (HER2) amplification was identified based on silver in situ hybridization (
Fig. 3C). The tumor cells were negative in ER and PR immunostainings (
Fig. 3D and E). These findings were similar to the initial pathology report of primary right breast cancer.
Fig. 3
Pathological findings of the brain biopsy. (A) The tumor cells showed a lack of tubule or gland formation, moderate nuclear pleomorphism, and frequent mitosis. (B) The tumor cells were homogeneous, dark circumferential pattern in HER immunostaining. (C) HER2 amplification was identified based on silver in situ hybridization. (D, E) The tumor cells were negative in estrogen receptor and progesterone receptor immunostainings.
Four months after completing whole brain radiation, spine MRI and brain MRI were conducted due to neuropathic pain in both legs and urinary incontinence. Leptomeningeal thickening and enhancement along spinal cord was detected, consistent with leptomeningeal seeding. Intrathecal methotrexate through Omaya valve was initiated with docetaxel, trastuzumab, and pertuzumab (THP) as a palliative systemic treatment. After two cycles of THP regimen and four weeks of twice-a-week scheduled intrathecal methotrexate, a partial response was confirmed and her neurological symptoms improved. Intrathecal methotrexate was discontinued due to intolerable toxicity of nausea and vomiting. Docetaxel was omitted after two cycles of THP due to intolerable neuropathic pain in both legs. She recently had her sixth cycles of trastuzumab and pertuzumab, while maintaining a stable neurological condition.
GENERAL INTRODUCTION OF THE DISEASE MANAGEMENT
Dr. Kabsoo Shin: The mainstay of treatment in early and locally advanced breast cancer includes surgery, radiation, endocrine therapy, and chemotherapy. The management of breast cancer patient with LFS follows the same principle as for the general population, but potential risk of radiation-induced malignancies and necessity of risk-reducing mastectomy need to be considered.
345
According to American Society of Clinical Oncology-American Society for Radiation Oncology-Society of Surgical Oncology guideline, mastectomy is a recommended surgical treatment over breast-conserving surgery for breast cancer patients with LFS to avoid adjuvant radiation, and postmastectomy radiotherapy should only be limited to patients at high risk of loco-regional recurrence.
15 Risk-reducing mastectomy needs to be discussed with patients considering high penetrance of LFS although there has been insufficient evidence that prophylactic mastectomy reduces the risk for breast cancer in LFS.
In this case, the patient had biopsy-confirmed cancer in the right breast and Paget disease with suspicious malignancy with multi-centric NME in the left breast. Initial clinical stage of the right Breast cancer was cT3N1 and the subtype was hormone-negative and HER2-positive. As in other cases of hormone-negative and HER2-positive locally advanced breast cancer, she was treated with neoadjuvant chemotherapy (TCHP regimen) first and adjuvant anti-HER2 treatment was given after pathological complete response was confirmed by bilateral mastectomy and SLNB.
DISCUSSION
Dr. Kabsoo Shin: Approximately 5% to 10% of breast cancer is associated with hereditary cancer syndromes.
6 LFS, a rare autosomal dominant hereditary syndrome, constitutes about 1% of hereditary breast cancers. LFS is related to germline pathogenic or likely pathogenic variant in the
TP53 gene, a central tumor suppressor gene regulating cell cycle and promoting DNA damage repair in response to stress signals.
7 The lifetime risk of developing cancer for LFS is nearly 100% for both females and males. The cumulative incidence of developing cancer is 50% by age 31 in females and age 46 in males. The difference between both sexes is mainly due to early incidence of breast cancer in females.
8
Somatic
TP53 mutations are considered as a potential poor prognostic marker in breast cancer.
9 On the other hand, the prognosis of breast cancer with germline
TP53 mutations is not well identified due to the rarity of the disease, but may be worse considering subsequent diagnoses of other primary cancers.
10 One study reported that
TP53 germline mutated breast cancer is associated with poor response to chemotherapy and radiation, and radiation-related sequelae also complicates the management of the disease.
11
Dr. Jeoungmin Lee: “When to suspect LFS in patients with breast cancer?”
Dr. Kabsoo Shin: When screening for LFS, the classic LFS criteria and the Chompret criteria are widely applied (as shown in
Table 1). By combining both criteria, 95% of patients with LFS can be detected. The most common malignancies in LFS is breast cancer, with a cumulative incidence of about 85% by age 60.
8 Breast cancer in LFS tends to develop at a much younger age compared to the general population.
12 It is estimated that about 3–8% of breast cancer patients diagnosed under age 30 without a family history have germline
TP53 mutation.
13 These evidences suggest that a substantial portion of early-onset breast cancer is caused by a germline
TP53 mutation, particularly when more common high-penetrance genes like
BRCA1/2 are negative. In such cases, even in the absence of a familial history, more efforts to find other pathogenic variants are needed. Either targeted TP53 gene mutation testing or multi-gene testing should be considered in this clinical condition.
4
Dr. Tae-Kyung Yoo: “Does germline TP53 mutated breast cancer have unique pathologic features?”
Dr. Jun Kang: Breast cancer in LFS has several pathological features compared to sporadic breast cancer. In a study evaluating histopathologic features of LFS showed that 60% of
TP53 germline mutated cases were HER2 positive, and co-expression of ER was seen in 44% of the cases.
14 Another retrospective study evaluated 21 breast cancer patients with LFS from South Korea and reported that around 50% of patients were HER2 positive, and 42% were ER or PR positive.
15 Considering around 20% of all breast cancers are HER2-positive, LFS breast cancer seems to be associated with HER2-positivity. Breast cancers in LFS also tend to have high tumor grade and similar morphologic features to somatic
TP53 mutated breast cancers.
14
Dr. Tae-Kyung Yoo: “What should be considered when managing breast cancer in LFS patients?”
Dr. Jieun Lee: When performing genetic testing, as in other cases of hereditary disease, comprehensive genetic counseling should be provided first, and patients should understand that diagnosis of the disease will have an impact on them and their families. After test result is confirmed, discussions about therapeutic plans, the medical risks based on the test results and family history, and notification of the results to family members should be considered.
14
When curative treatment is indicated for breast cancer patients with LFS, mastectomy is a recommended surgical treatment to avoid additional radiation.
5 The option of contralateral risk-reducing mastectomy needs to be considered and discussed with patients. Radiotherapy is best avoided due to the risk of secondary cancer. When other treatment options are not applicable, after assessing the risk and benefit through a multidisciplinary discussion, radiotherapy could be applied with adaptation in a case-by-case manner.
16
After curative treatment, surveillance of both recurrence and other primary cancer is important. Based on Toronto protocol and National Comprehensive Cancer Network guideline, whole-body MRI (WBMRI) and brain MRI need to be performed as a surveillance in LFS due to high risk of malignancies.
1 From age 20 to 75 years, annual breast MRI and annual WBMRI are recommended alternately at 6-month intervals. From age 25, colonoscopy is recommended every 2 years. Annual comprehensive physical exam including neurologic and dermatologic exam and patient education are also recommended for surveillance of skin and other conditions. In this case, the patient followed recommended surveillance protocol including annual WBMRI and brain MRI alternating with annual breast MRI.
ACKNOWLEDGMENTS
The Case Conference section is prepared from monthly case conference of Department of Internal Medicine, the Catholic University of Korea College of Medicine, Seoul, Korea
References
1. Kumamoto T, Yamazaki F, Nakano Y, Tamura C, Tashiro S, Hattori H, et al. Medical guidelines for Li-Fraumeni syndrome 2019, version 1.1. Int J Clin Oncol. 2021; 26(12):2161–2178. PMID:
34633580.

2. Chompret A, Brugières L, Ronsin M, Gardes M, Dessarps-Freichey F, Abel A, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer. 2000; 82(12):1932–1937. PMID:
10864200.
3. Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022; 20(6):691–722. PMID:
35714673.
4. Weiss JM, Gupta S, Burke CA, Axell L, Chen LM, Chung DC, et al. NCCN guidelines(r) insights: genetic/familial high-risk assessment: colorectal, version 1.2021. J Natl Compr Canc Netw. 2021; 19(10):1122–1132. PMID:
34666312.
5. Trombetta MG, Dragun A, Mayr NA, Pierce LJ. Astro radiation therapy summary of the asco-astro-sso guideline on management of hereditary breast cancer. Pract Radiat Oncol. 2020; 10(4):235–242. PMID:
32471709.
6. Tung N, Lin NU, Kidd J, Allen BA, Singh N, Wenstrup RJ, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016; 34(13):1460–1468. PMID:
26976419.

7. Sidransky D, Tokino T, Helzlsouer K, Zehnbauer B, Rausch G, Shelton B, et al. Inherited p53 gene mutations in breast cancer. Cancer Res. 1992; 52(10):2984–2986. PMID:
1581912.
8. Gonzalez KD, Noltner KA, Buzin CH, Gu D, Wen-Fong CY, Nguyen VQ, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol. 2009; 27(8):1250–1256. PMID:
19204208.

9. Silwal-Pandit L, Vollan HK, Chin SF, Rueda OM, McKinney S, Osako T, et al. TP53 mutation spectrum in breast cancer is subtype specific and has distinct prognostic relevance. Clin Cancer Res. 2014; 20(13):3569–3580. PMID:
24803582.

10. Mai PL, Best AF, Peters JA, DeCastro RM, Khincha PP, Loud JT, et al. Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer. 2016; 122(23):3673–3681. PMID:
27496084.

11. Kappel S, Janschek E, Wolf B, Rudas M, Teleky B, Jakesz R, et al. TP53 germline mutation may affect response to anticancer treatments: analysis of an intensively treated Li-Fraumeni family. Breast Cancer Res Treat. 2015; 151(3):671–678. PMID:
25981898.

12. Masciari S, Dillon DA, Rath M, Robson M, Weitzel JN, Balmana J, et al. Breast cancer phenotype in women with TP53 germline mutations: a Li-Fraumeni syndrome consortium effort. Breast Cancer Res Treat. 2012; 133(3):1125–1130. PMID:
22392042.

13. Kamihara J, Rana HQ, Garber JE. Germline TP53 mutations and the changing landscape of Li-Fraumeni syndrome. Hum Mutat. 2014; 35(6):654–662. PMID:
24706533.

14. Kuba MG, Lester SC, Bowman T, Stokes SM, Taneja KL, Garber JE, et al. Histopathologic features of breast cancer in Li-Fraumeni syndrome. Mod Pathol. 2021; 34(3):542–548. PMID:
32636452.

15. Alyami H, Yoo TK, Cheun JH, Lee HB, Jung SM, Ryu JM, et al. Clinical features of breast cancer in South Korean patients with germline tp53 gene mutations. J Breast Cancer. 2021; 24(2):175–182. PMID:
33818021.

16. Thariat J, Chevalier F, Orbach D, Ollivier L, Marcy PY, Corradini N, et al. Avoidance or adaptation of radiotherapy in patients with cancer with Li-Fraumeni and heritable TP53-related cancer syndromes. Lancet Oncol. 2021; 22(12):e562–e574. PMID:
34856153.









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download