1. Beaupre GS, Lew HL. Bone-density changes after stroke. Am J Phys Med Rehabil. 2006; 85:464–72.

2. Tinetti ME, Liu WL, Claus EB. Predictors and prognosis of inability to get up after falls among elderly persons. JAMA. 1993; 269:65–70.

3. Kanis J, Oden A, Johnell O. Acute and long-term increase in fracture risk after hospitalization for stroke. Stroke. 2001; 32:702–6.

4. Pasco JA, Henry MJ, Korn S, Nicholson GC, Kotowicz MA. Morphometric vertebral fractures of the lower thoracic and lumbar spine, physical function and quality of life in men. Osteoporos Int. 2009; 20:787–92.

5. Carda S, Cisari C, Invernizzi M, Bevilacqua M. Osteoporosis after stroke: a review of the causes and potential treatments. Cerebrovasc Dis. 2009; 28:191–200.

6. del Puente A, Pappone N, Mandes MG, Mantova D, Scarpa R, Oriente P. Determinants of bone mineral density in immobilization: a study on hemiplegic patients. Osteoporos Int. 1996; 6:50–4.

7. Hamdy RC, Moore SW, Cancellaro VA, Harvill LM. Long-term effects of strokes on bone mass. Am J Phys Med Rehabil. 1995; 74:351–6.

8. Ramnemark A, Nyberg L, Lorentzon R, Englund U, Gustafson Y. Progressive hemiosteoporosis on the paretic side and increased bone mineral density in the nonparetic arm the first year after severe stroke. Osteoporos Int. 1999; 9:269–75.

9. Kim HD, Kim SH, Kim DK, Jeong HJ, Sim YJ, Kim GC. Change of bone mineral density and relationship to clinical parameters in male stroke patients. Ann Rehabil Med. 2016; 40:981–8.

10. Lee HY, Park JH, Lee H, Kim TW, Yoo SD. Does hip bone density differ between paretic and non-paretic sides in hemiplegic stroke patients? and its relationship with physical impairment. J Bone Metab. 2020; 27:237–46.

11. Haruyama K, Kawakami M, Otsuka T. Effect of core stability training on trunk function, standing balance, and mobility in stroke patients. Neurorehabil Neural Repair. 2017; 31:240–9.

12. McDonnell P, McHugh PE, O'Mahoney D. Vertebral osteoporosis and trabecular bone quality. Ann Biomed Eng. 2007; 35:170–89.

13. Chen H, Zhou X, Fujita H, Onozuka M, Kubo KY. Age-related changes in trabecular and cortical bone microstructure. Int J Endocrinol. 2013; 2013:213234.

14. Legrand E, Chappard D, Pascaretti C, Duquenne M, Krebs S, Rohmer V, et al. Trabecular bone microarchitecture, bone mineral density, and vertebral fractures in male osteoporosis. J Bone Miner Res. 2000; 15:13–9.

15. Dalle Carbonare L, Giannini S. Bone microarchitecture as an important determinant of bone strength. J Endocrinol Invest. 2004; 27:99–105.

16. Jørgensen L, Jacobsen BK, Wilsgaard T, Magnus JH. Walking after stroke: does it matter? Changes in bone mineral density within the first 12 months after stroke. A longitudinal study. Osteoporos Int. 2000; 11:381–7.

17. Demirbag D, Ozdemir F, Kokino S, Berkarda S. The relationship between bone mineral density and immobilization duration in hemiplegic limbs. Ann Nucl Med. 2005; 19:695–700.

18. Arceo-Mendoza RM, Camacho PM. Postmenopausal osteoporosis: latest guidelines. Endocrinol Metab Clin North Am. 2021; 50:167–78.
19. Vilaca T, Eastell R, Schini M. Osteoporosis in men. Lancet Diabetes Endocrinol. 2022; 10:273–83.

20. Rinonapoli G, Ruggiero C, Meccariello L, Bisaccia M, Ceccarini P, Caraffa A. Osteoporosis in men: a review of an underestimated bone condition. Int J Mol Sci. 2021; 22:2105.

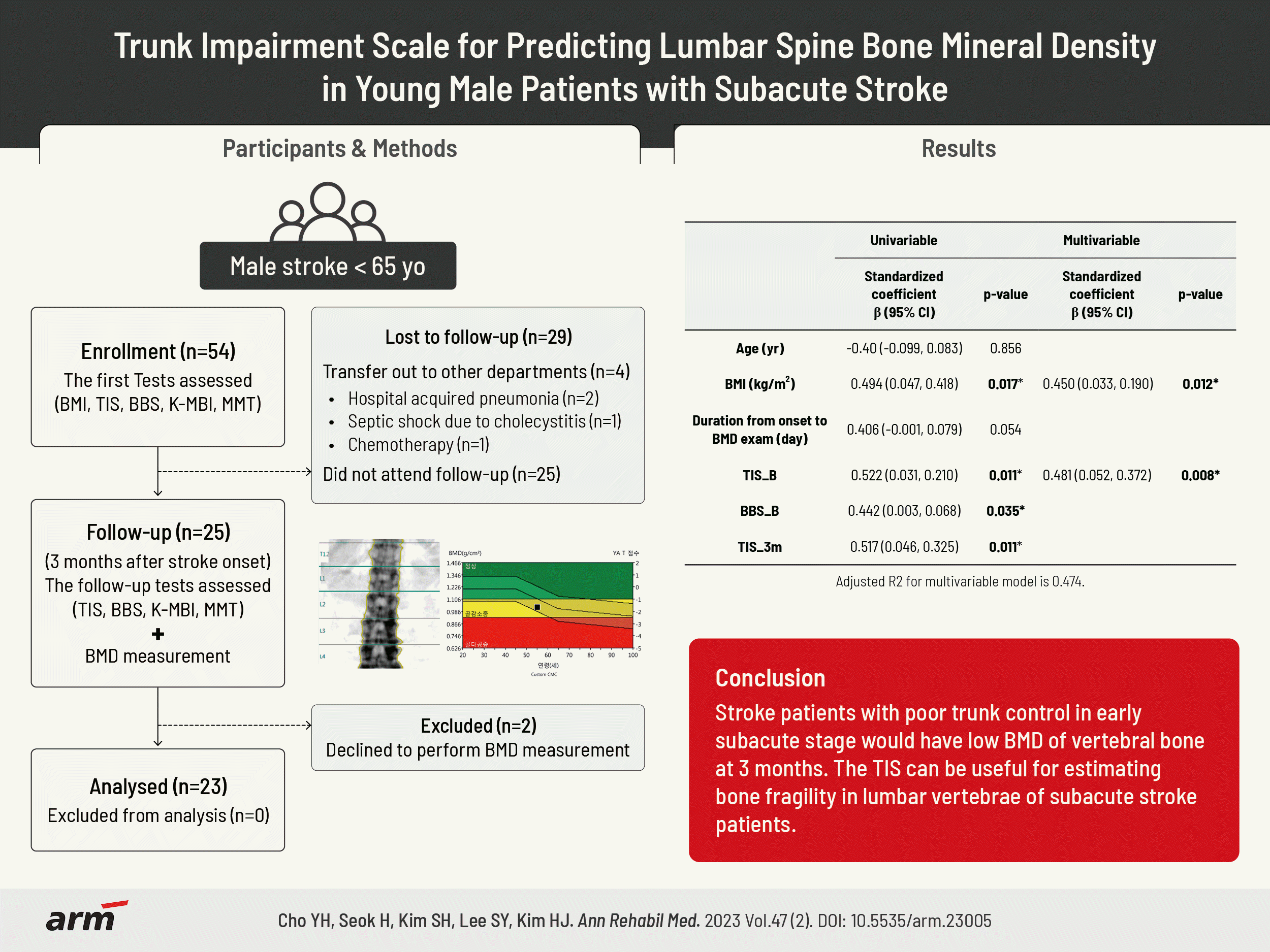
21. Verheyden G, Nieuwboer A, Mertin J, Preger R, Kiekens C, De Weerdt W. The Trunk Impairment Scale: a new tool to measure motor impairment of the trunk after stroke. Clin Rehabil. 2004; 18:326–34.

22. Kleerekoper M, Nelson DA. Which bone density measurement? J Bone Miner Res. 1997; 12:712–4.

23. Shuhart CR, Yeap SS, Anderson PA, Jankowski LG, Lewiecki EM, Morse LR, et al. Executive summary of the 2019 ISCD position development conference on monitoring treatment, DXA cross-calibration and least significant change, spinal cord injury, peri-prosthetic and orthopedic bone health, transgender medicine, and pediatrics. J Clin Densitom. 2019; 22:453–71.

24. Akoglu H. User’s guide to correlation coefficients. Turk J Emerg Med. 2018; 18:91–3.

25. Jang A, Bae CH, Han SJ, Bae H. Association between length of stay in the intensive care unit and sarcopenia among hemiplegic stroke patients. Ann Rehabil Med. 2021; 45:49–56.

26. Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short-term disuse: implications for agerelated sarcopenia. Ageing Res Rev. 2013; 12:898–906.

27. Krabak B, Kennedy DJ. Functional rehabilitation of lumbar spine injuries in the athlete. Sports Med Arthrosc Rev. 2008; 16:47–54.

28. Oftadeh R, Perez-Viloria M, Villa-Camacho JC, Vaziri A, Nazarian A. Biomechanics and mechanobiology of trabecular bone: a review. J Biomech Eng. 2015; 137:0108021–01080215.

29. Banijamali SM, Oftadeh R, Nazarian A, Goebel R, Vaziri A, Nayeb-Hashemi H. Effects of different loading patterns on the trabecular bone morphology of the proximal femur using adaptive bone remodeling. J Biomech Eng. 2015; 137:011011.
30. Osterhoff G, Morgan EF, Shefelbine SJ, Karim L, McNamara LM, Augat P. Bone mechanical properties and changes with osteoporosis. Injury. 2016; 47(Suppl 2):S11–20.

31. Fukuoka H, Nishimura Y, Haruna M, Suzuki Y, Oyama K, Igawa S, et al. Effect of bed rest immobilization on metabolic turnover of bone and bone mineral density. J Gravit Physiol. 1997; 4:S75–81.
32. Burr DB, Robling AG, Turner CH. Effects of biomechanical stress on bones in animals. Bone. 2002; 30:781–6.

33. Ehrlich PJ, Noble BS, Jessop HL, Stevens HY, Mosley JR, Lanyon LE. The effect of in vivo mechanical loading on estrogen receptor alpha expression in rat ulnar osteocytes. J Bone Miner Res. 2002; 17:1646–55.

34. Tan SD, Bakker AD, Semeins CM, Kuijpers-Jagtman AM, Klein-Nulend J. Inhibition of osteocyte apoptosis by fluid flow is mediated by nitric oxide. Biochem Biophys Res Commun. 2008; 369:1150–4.

35. Bansod YD, Kebbach M, Kluess D, Bader R, van Rienen U. Computational analysis of bone remodeling in the proximal tibia under electrical stimulation considering the piezoelectric properties. Front Bioeng Biotechnol. 2021; 9:705199.

36. Lazoura O, Groumas N, Antoniadou E, Papadaki PJ, Papadimitriou A, Thriskos P, et al. Bone mineral density alterations in upper and lower extremities 12 months after stroke measured by peripheral quantitative computed tomography and DXA. J Clin Densitom. 2008; 11:511–7.
37. Ramnemark A, Nyberg L, Lorentzon R, Olsson T, Gustafson Y. Hemiosteoporosis after severe stroke, independent of changes in body composition and weight. Stroke. 1999; 30:755–60.

38. Palle S, Vico L, Bourrin S, Alexandre C. Bone tissue response to four-month antiorthostatic bedrest: a bone histomorphometric study. Calcif Tissue Int. 1992; 51:189–94.

39. Ishiwatari M, Tani M, Isayama R, Honaga K, Hayakawa M, Takakura T, et al. Prediction of gait independence using the Trunk Impairment Scale in patients with acute stroke. Ther Adv Neurol Disord. 2022; 15:17562864221140180.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download