Abstract
Since the coronavirus disease 2019 (COVID-19) outbreak caused by the severe acute respiratory syndrome-coronavirus-2 virus (SARS-CoV-2), various complications have been reported. Although most COVID-19 cases exhibited flu-like symptoms, COVID-19 may dysregulate the immune response and promote overwhelming levels of inflammation in some patients. Inflammatory bowel disease (IBD) is caused by dysregulated or inappropriate immune responses to environmental factors in a genetically susceptible host, and a SARS-CoV-2 infection may act as a possible cause of IBD. This paper describes two pediatric patients who developed Crohn’s disease following a SARS-CoV-2 infection. They were previously healthy before the SARS-CoV-2 infection. On the other hand, they started to develop fever and gastrointestinal symptoms several weeks after recovery from the infection. They were diagnosed with Crohn’s disease by imaging and endoscopic studies, and their symptoms improved after treatment with steroids and azathioprine. This paper suggests that a SARS-CoV-2 infection may trigger IBD in predisposed patients.
Coronavirus disease 2019 (COVID-19), a novel infectious disease, caused by the severe acute respiratory syndrome-coronavirus- 2 (SARS-CoV-2) virus, was declared a pandemic by the World Health Organization on March 11, 2020.1 COVID-19 can exhibit various symptoms, ranging from asymptomatic to severe clinical symptoms. In some cases, it may trigger immune dysregulation and impact the onset of various immunological diseases. Inflammatory bowel disease (IBD) is an immunological disease, and there are several reports of IBD following COVID-19.2,3 Tursi and Nenna4 reported a 47-year-old female who developed de novo Crohn’s disease following COVID-19. On the other hand, data determining the association between COVID-19 and IBD are scarce. This paper reports two pediatric cases of Crohn’s disease triggered soon after recovery from the SARS-CoV-2 infection.
The Institutional Review Board (IRB) of Bundang Jesaeng Medical Hospital approved this study (IRB No. 2022-07-011). The requirement of informed consent for publishing was waived.
A 17-year-old male without any specific medical history or family history of IBD, a non-smoker, presented to the authors’ hospital with a sore throat, cough, and fever. His nasopharyngeal rapid antigen test was positive for SARS-CoV-2. He had no gastrointestinal symptoms, such as abdominal pain, diarrhea, or vomiting, and did not demonstrate any signs or symptoms of pneumonia, such as dyspnea or desaturation; therefore, admission was not required. He received antipyretics and was advised to undergo home isolation. The symptoms improved within a week.
After two weeks of recovery, he developed nausea, daily vomiting, watery diarrhea more than 10 times a day, moderate to severe periumbilical pain, and intermittent fever of up to 38.3℃. His oral intake decreased, and he lost 6 kg of body weight. He denied any previous episodes of similar symptoms. He was hospitalized 25 days after the COVID-19 diagnosis, with a provisional diagnosis of acute gastroenteritis. A physical examination revealed direct tenderness in the periumbilical area. The laboratory results were as follows: hemoglobin, 14.5 g/dL; platelet count, 367,000/mm3; white blood cell count, 16,400/mm3 (neutrophils 76.6%, lymphocytes 10.1%); erythrocyte sedimentation rate, 68 mm/h; serum total protein level, 7.3 g/dL; serum albumin level, 4.0 g/dL; and C-reactive protein, 17.62 mg/dL. Plain radiography of the abdomen showed no abnormalities, but abdominal ultrasonography revealed diffuse ileal bowel wall thickening, suggesting enteritis.
The patient received intravenous ceftriaxone for four days, but the symptoms worsened. He developed multiple oral ulcers that caused severe pain. He lost an additional 6 kg of weight during hospitalization. Diarrhea and fever persisted despite changing the antibiotics to piperacillin/tazobactam for another five days. Stool culture and PCR revealed no pathogens; however, the fecal calprotectin level was elevated to 5,378.46 mg/g.
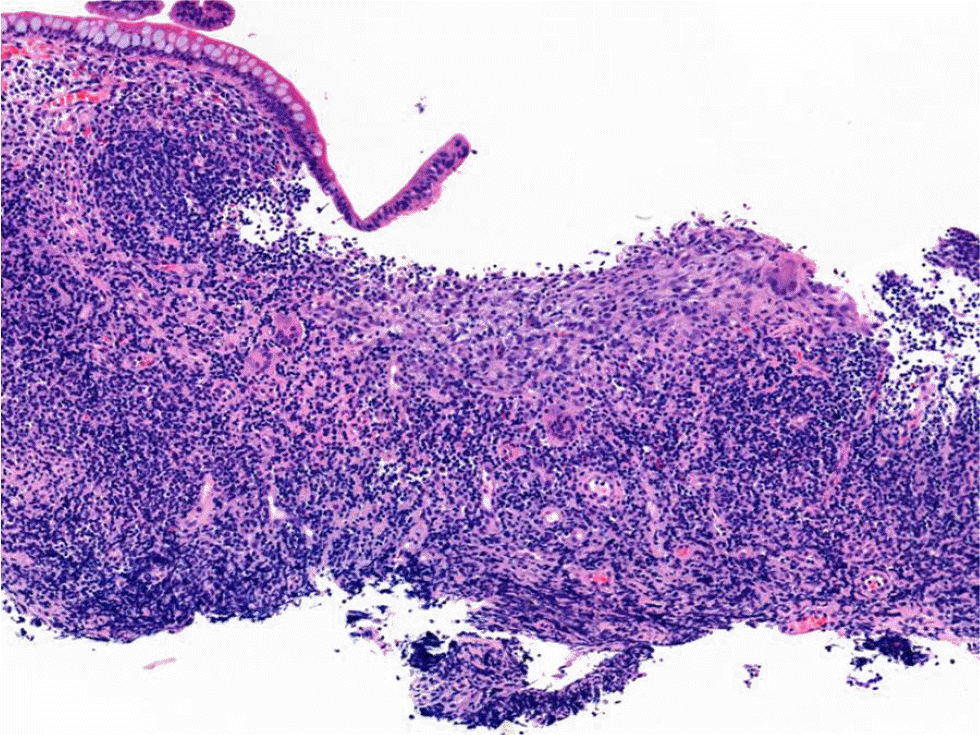
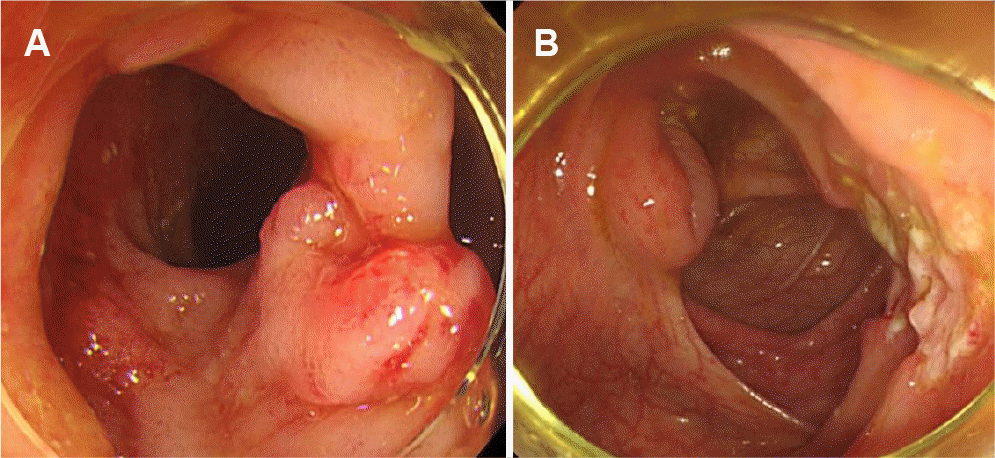
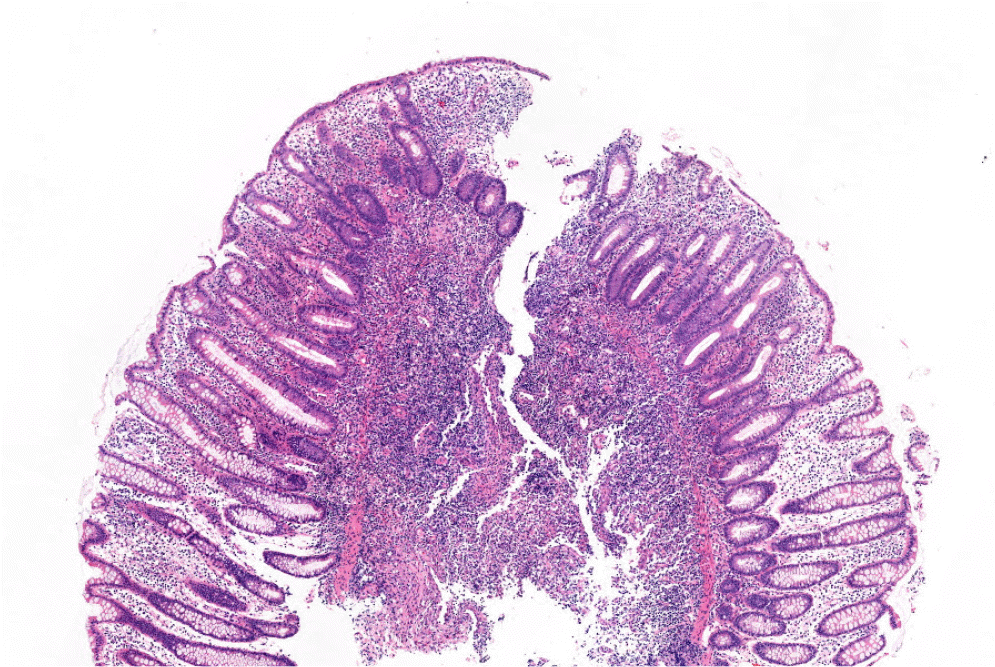
IBD was suspected, and further evaluations were performed. Magnetic resonance enterography revealed segmental wall thickening of the distal ileum with some deep mucosal enhancement (Fig. 1). Esophagogastroduodenoscopy revealed a few small ulcers in the distal esophagus (Fig. 2A). Ileocolonoscopy revealed edematous mucosa with multiple erosions, prominent lymphoid follicles in the terminal ileum (Fig. 2B), and a small ulcer in the ascending colon (Fig. 2C). A histopathological examination of the esophagus showed chronic esophagitis with focal detached fibrinosuppurative exudate. The terminal ileum revealed chronic granulomatous inflammation with a few multinucleated giant cells (Fig. 3). Based on the clinicopathological findings, the patient was diagnosed with Crohn’s disease according to the Montreal classification, A2, L3+L4, B1.
Intravenous methylprednisolone (40 mg) was administered daily because he could not swallow food due to severe oral pain. After the oral ulcers improved, the medication was changed to oral prednisolone, and he was discharged with an improved general condition. After confirming the histopathologic results, 100 mg of azathioprine daily was added, and prednisolone tapering was started. His Pediatric Crohn’s Disease Activity Index score improved from 35 to 10. The timing of maintenance therapy will be assessed based on the disease activity during follow-up.
An 11-year-old male without any specific medical history or family history of IBD, a non-smoker, developed fever and tested positive in a nasopharyngeal rapid antigen test for SARS-CoV-2. At that time, he did not have any gastrointestinal symptoms, such as abdominal pain, diarrhea, or vomiting, or any symptoms of pneumonia. He was isolated at home without special treatment. The symptoms improved within a few days.
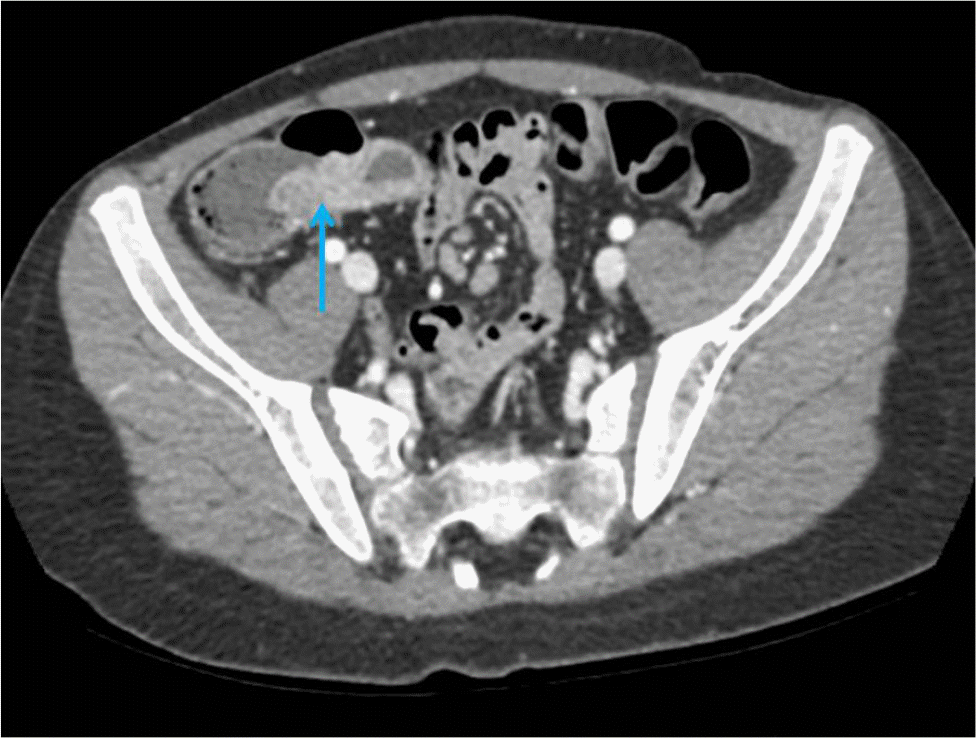
Recurrent moderate to severe periumbilical pain with watery diarrhea at least twice a day occurred a few weeks after being released from quarantine due to COVID-19. The symptoms worsened over two months, and he lost 4 kg of body weight. Subsequently, he developed fever and visited the authors’ clinic. The abdomen was soft and flat, with no palpable mass, but he had tenderness on the mid-lower abdomen. The laboratory results were as follows: hemoglobin, 12.5 g/dL; platelet count, 449,000/mm3; white blood cell count, 13,400/mm3 (neutrophils 70.7%, lymphocytes 20.7%); erythrocyte sedimentation rate, 55 mm/h; serum total protein level, 7.9 g/dL; serum albumin level, 4.2 g/dL; and C-reactive protein, 3.1 mg/dL. Plain radiography of the abdomen revealed no abnormalities, whereas abdominal computed tomography demonstrated wall thickening of the terminal ileum, suggesting enteritis (Fig. 4). The stool culture and PCR did not reveal any pathogens; however, the fecal calprotectin level was elevated up to 3,498.72 mg/g.
IBD was suspected. Therefore, he was hospitalized to receive intravenous antibiotics, and endoscopic examinations were performed. Esophagogastroduodenoscopy showed no abnormal findings, while ileocolonoscopy revealed ileocecal valve deformity with mucosal edema and ulcerations (Fig. 5A) and a large ulcer with cobblestone appearance in the cecum (Fig. 5B). The histopathology of the colon revealed focal aphthous ulcer formation with acute and chronic inflammation, and inflamed granulation tissue formation (Fig. 6).
Thus, the patient was diagnosed with Crohn’s disease according to Montreal classification, A1, L2, B1. He was started on 40 mg of oral prednisolone, and the symptoms improved. His Pediatric Crohn’s Disease Activity Index score decreased from 40 to 12.5. After discharge, 50 mg of azathioprine was added, and prednisolone tapering was started while monitoring for a flare-up of the symptoms.
In these two cases, both patients were previously healthy aside from a recent SARS-CoV-2 infection, and new onset Crohn’s disease was reported several weeks after recovery from the SARS-CoV-2 infection. Both cases had COVID-19-induced symptoms that were sufficiently mild to require no special hospitalization treatment and had no specific gastrointestinal symptoms at the time, with symptoms developing several weeks after recovery, eventually leading to the diagnosis of Crohn’s disease. To the best of the authors’ knowledge, this is the first report of Crohn’s disease triggered after COVID-19 in pediatric patients.
COVID-19 can interrupt self-tolerance and trigger autoimmune responses through cross-reactivity with the host cells.5 Viruses share immune responses with autoimmune diseases but can break immunological tolerance through various mechanisms, including molecular mimicry, bystander activation, and epitope spreading.6 Cases of cold agglutinin syndrome, autoimmune hemolytic anemia, and Guillain–Barré syndrome have been reported as autoimmune diseases that occur after SARS-CoV-2 infection.7-9 IBD is also an immunological disease, and an abnormality in intestinal mucosal immunoregulation may be paramount in the pathogenesis of IBD, involving the activation of cytokines and triggering a cascade of reactions that result in bowel inflammation.10 COVID-19 may trigger the development of IBD, particularly through its dysregulation effects on the immune system.2,3
Although COVID-19 is generally milder in children than adults, a rare pediatric syndrome known as a multisystem inflammatory syndrome in children (MIS-C) has been reported globally.11 MIS-C is a serious post-infectious syndrome that causes a cytokine storm and multiorgan dysfunction. The clinical presentation of MIS-C is diverse, and gastrointestinal manifestations are often the most prominent features of MIS-C.12 Severe gastrointestinal manifestations of MIS-C may mimic the symptoms of IBD, or IBD may present with concurrent MIS-C.13,14 Thus, distinguishing MIS-C from the exacerbations of chronic inflammatory conditions, including IBD, may be challenging. In this study, the patients had severe gastrointestinal symptoms, but they did not have any other extraintestinal manifestations, such as rash, conjunctivitis, coagulopathy, cardiovascular, and neurologic symptoms. The histological features of intestinal inflammation in MIS-C appear different from those in IBD, which may help differentiate IBD from MIS-C. The features of MIS-C may include venous microthrombi, arteritis, and necrotizing lymphadenitis.15 In these cases, chronic granulomatous inflammation was noted, suggesting Crohn’s disease rather than MIS-C. Limited information is available regarding whether MIS-C may trigger IBD onset. Although there may be a possible mechanism of potential susceptibility to autoimmune disease,16 no data on the clinical course of this condition has been published. Further studies on the impact of COVID-19 and MIS-C on the manifestation of autoimmune diseases are warranted.
The pathophysiology of IBD is closely linked to complex and mutual interactions between host genetics, environmental factors, gut dysbiosis, and mucosal immune systems.16 The commensal microbiota ecosystem in the gut is dynamic and can be regulated by invading viruses to facilitate stimulatory or suppressive responses.17 The human intestine expresses high levels of angiotensin-converting enzyme 2 and transmembrane serine protease, which are required for SARS-CoV-2 to enter the cells.18 Thus, SARS-CoV-2 can invade the gastrointestinal tract, and a SARS-CoV-2 infection in the intestine can cause changes in the microbiota, leading to intestinal dysbiosis.19 Dysbiosis increases the intestinal permeability and induces inflammatory responses by regulating the expression of inflammatory genes, leading to intestinal inflammation.20 Further research is needed to determine if intestinal dysbiosis caused by COVID-19 can trigger de novo IBD.
In conclusion, COVID-19 can cause immune dysregulation or MIS-C in susceptible individuals, leading to chronic inflammatory conditions. Although it is unclear if COVID-19 can cause IBD, the possible mechanisms of the disease need to be considered. Further studies will be needed to clarify the correlation between COVID-19 and IBD, as well as research on the prevention and treatment of the disease.
REFERENCES
1. Li Q, Guan X, Wu P, et al. 2020; Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 382:1199–1207. DOI: 10.1056/NEJMoa2001316. PMID: 31995857. PMCID: PMC7121484.
2. Senthamizhselvan K, Ramalingam R, Mohan P, Kavadichanda C, Badhe B, Hamide A. 2021; De novo Crohn's disease triggered after COVID-19: Is COVID-19 more than an infectious disease? ACG Case Rep J. 8:e00652. DOI: 10.14309/crj.0000000000000652. PMID: 34476279. PMCID: PMC8386903.
3. Thongtan T, Deb A, Islam S. 2021; De novo inflammatory bowel disease is a potential post-acute sequela of SARS-CoV-2 infection. Southwest Respir Crit Care Chron. 9:35–39. DOI: 10.12746/swrccc.v9i41.913.
4. Tursi A, Nenna R. 2022; COVID-19 as a trigger for de novo Crohn's disease. Inflamm Bowel Dis. 28:e76–e77. DOI: 10.1093/ibd/izab298. PMID: 35657373. PMCID: PMC8690164.
5. Liu Y, Sawalha AH, Lu Q. 2021; COVID-19 and autoimmune diseases. Curr Opin Rheumatol. 33:155–162. DOI: 10.1097/BOR.0000000000000776. PMID: 33332890. PMCID: PMC7880581.
6. Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, Yassine HM. 2019; Viruses and autoimmunity: A review on the potential interaction and molecular mechanisms. Viruses. 11:762. DOI: 10.3390/v11080762. PMID: 31430946. PMCID: PMC6723519.
7. Jensen CE, Wilson S, Thombare A, Weiss S, Ma A. 2020; Cold agglutinin syndrome as a complication of Covid-19 in two cases. Clin Infect Pract. 7:100041. DOI: 10.1016/j.clinpr.2020.100041. PMID: 32924007. PMCID: PMC7480768.
8. Patil NR, Herc ES, Girgis M. 2022; Cold agglutinin disease and autoimmune hemolytic anemia with pulmonary embolism as a presentation of COVID-19 infection. Hematol Oncol Stem Cell Ther. 15:213–216.
9. Gigli GL, Vogrig A, Nilo A, et al. 2020; HLA and immunological features of SARS-CoV-2-induced Guillain-Barré syndrome. Neurol Sci. 41:3391–3394. DOI: 10.1007/s10072-020-04787-7. PMID: 33006723. PMCID: PMC7530349.
10. Zhang YZ, Li YY. 2014; Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 20:91–99. DOI: 10.3748/wjg.v20.i1.91. PMID: 24415861. PMCID: PMC3886036.
11. Levin M. 2020; Childhood multisystem inflammatory syndrome - A new challenge in the pandemic. N Engl J Med. 383:393–395. DOI: 10.1056/NEJMe2023158. PMID: 32598829. PMCID: PMC7346677.
12. Hoste L, Van Paemel R, Haerynck F. 2021; Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr. 180:2019–2034. DOI: 10.1007/s00431-021-03993-5. PMID: 33599835. PMCID: PMC7890544.
13. Sweeny KF, Zhang YJ, Crume B, Martz CA, Blessing MM, Kahn SA. 2021; Inflammatory bowel disease presenting with concurrent COVID-19 multisystem inflammatory syndrome. Pediatrics. 147:e2020027763. DOI: 10.1542/peds.2020-027763. PMID: 33414238. PMCID: PMC8015148.
14. Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis KG. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children that is related to coronavirus disease 2019: A single center experience of 44 cases. Gastroenterology. 2020; 159:1571–1574.e2. DOI: 10.1053/j.gastro.2020.05.079. PMID: 32505742. PMCID: PMC7270806.
15. Sahn B, Eze OP, Edelman MC, et al. 2021; Features of intestinal disease associated with COVID-related multisystem inflammatory syndrome in children. J Pediatr Gastroenterol Nutr. 72:384–387. DOI: 10.1097/MPG.0000000000002953. PMID: 32969960. PMCID: PMC7901530.
16. Krawiec P, Opoka-Winiarska V, Pac-Kożuchowska E. 2022; Is it inflammatory bowel disease flare or pediatric inflammatory multisystem syndrome temporally associated with COVID-19? J Clin Med. 11:2765. DOI: 10.3390/jcm11102765. PMID: 35628892. PMCID: PMC9143677.
17. Li N, Ma WT, Pang M, Fan QL, Hua JL. 2019; The commensal microbiota and viral infection: A comprehensive review. Front Immunol. 10:1551. DOI: 10.3389/fimmu.2019.01551. PMID: 31333675. PMCID: PMC6620863.
18. Zhang H, Kang Z, Gong H, et al. 2020; Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 69:1010–1018. DOI: 10.1136/gutjnl-2020-320953. PMCID: PMC7211082.
19. Din AU, Mazhar M, Waseem M, et al. 2021; SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed Pharmacother. 133:110947. DOI: 10.1016/j.biopha.2020.110947. PMID: 33197765. PMCID: PMC7657099.
20. Ahmed I, Roy BC, Khan SA, Septer S, Umar S. 2016; Microbiome, metabolome and inflammatory bowel disease. Microorganisms. 4:20. DOI: 10.3390/microorganisms4020020. PMID: 27681914. PMCID: PMC5029486.
Fig. 1
Magnetic resonance enterography shows segmental wall thickening of the distal ileum with some deep mucosal enhancement. (yellow arrows).
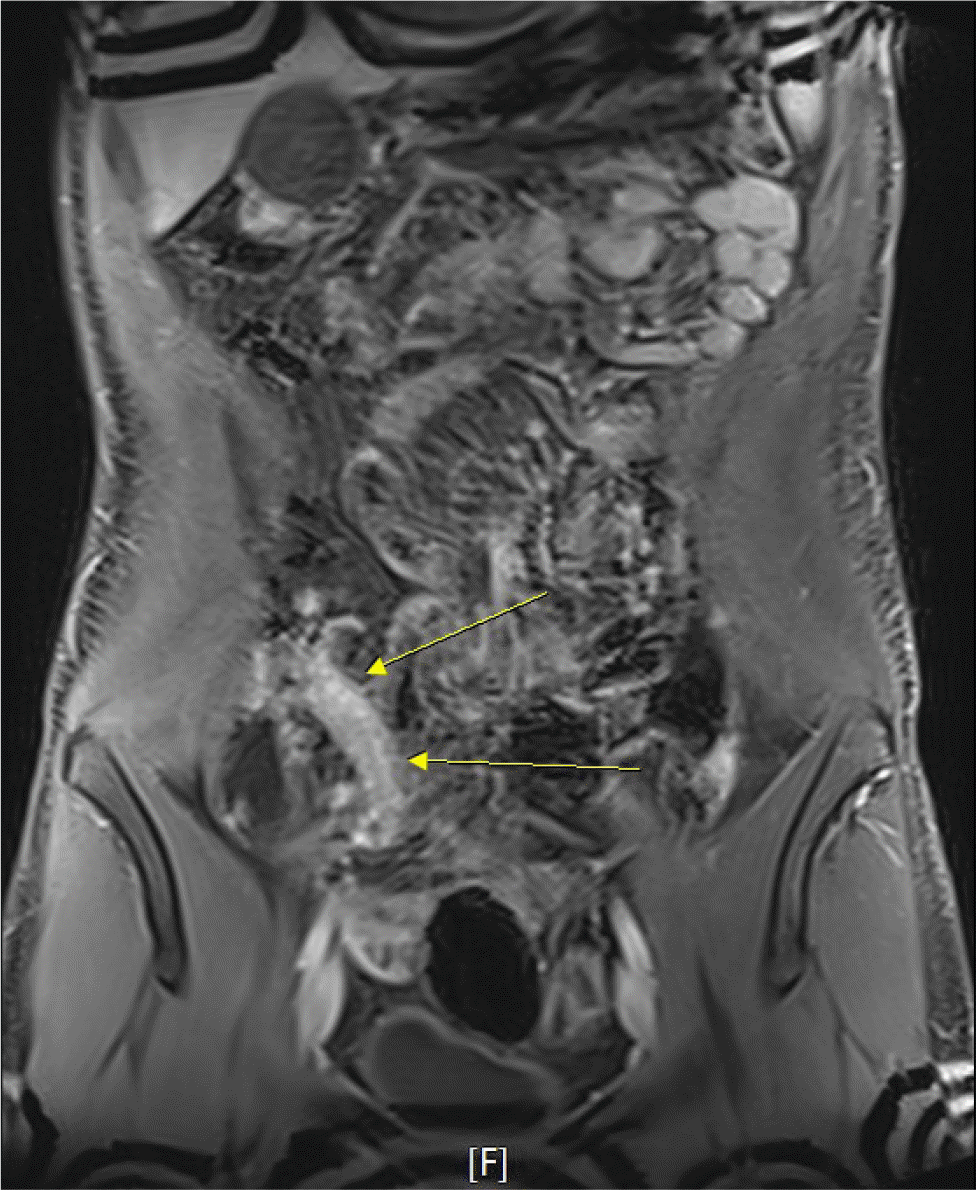
Fig. 2
Esophagogastroduodenoscopy shows a few small ulcers in the distal esophagus (A), and ileocolonoscopy shows edematous mucosa with multiple erosions, prominent lymphoid follicles in the terminal ileum (B), and a small ulcer in the ascending colon (C).
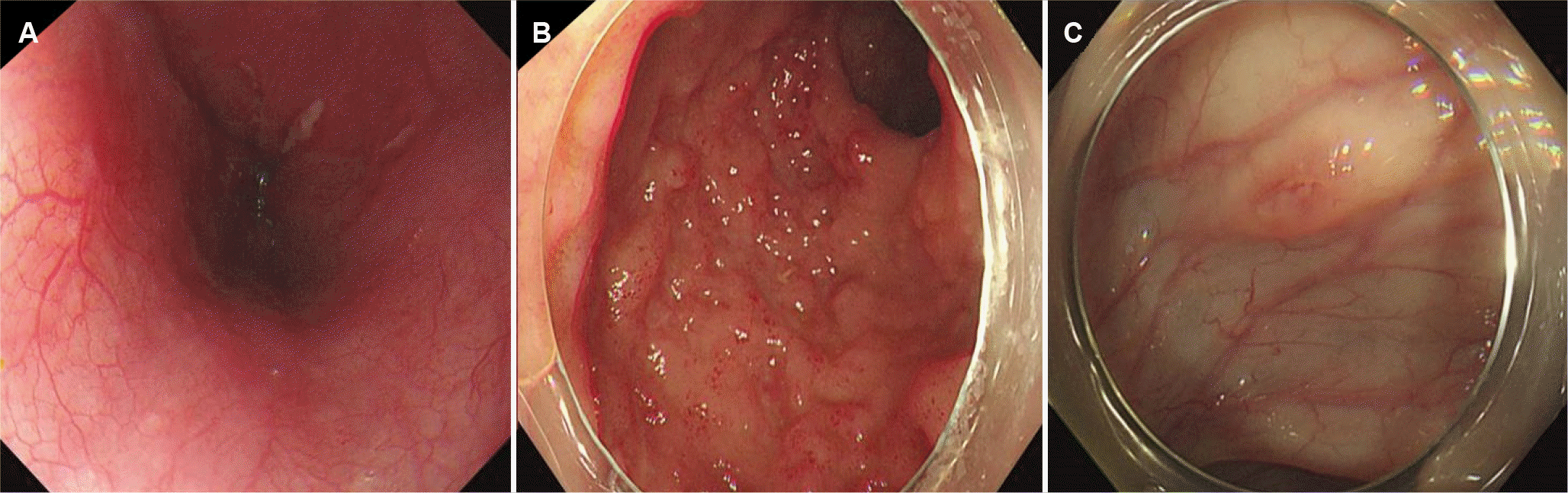
Fig. 3
Microscopic finding of the terminal ileum shows an ill-defined granuloma with multinucleated giant cells and no necrosis associated with chronic inflammatory cells (H&E, ×100).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download