Abstract
Background/Aims
A percutaneous gastrostomy can be placed either endoscopically (percutaneous endoscopic gastrostomy, PEG) or radiologically (radiologically-inserted gastrostomy, RIG). However, there is no consistent evidence of the safety and efficacy of PEG compared to RIG. Recently, 30-day mortality has become considered as the most important surrogate index for evaluating the safety and efficacy of percutaneous gastrostomy. The aim of this meta-analysis was to compare the 30-day mortality rates between PEG and RIG.
Methods
Major electronic databases (MEDLINE, Embase, Scopus, and Cochrane library) were queried for comparative studies on the two insertion techniques of gastrostomy among adults with swallowing disturbance. The primary outcome was the 30-day mortality rate after gastrostomy insertion. Forest and funnel plots were generated for outcomes using STATA version 14.0.
Results
Fifteen studies (n=2,183) met the inclusion criteria. PEG was associated with a lower risk of 30-day mortality after tube placement compared with RIG (odds ratio, 0.60; 95% confidence interval [CI], 0.38–0.94; P=0.026). The pooled prevalence of 30-day mortality of PEG was 5.5% (95% CI, 4.0%–6.9%) and that of RIG was 10.5% (95% CI, 6.8%–14.3%). No publication bias was noted.
Conclusions
The present meta-analysis demonstrated that PEG is associated with a lower probability of 30-day mortality compared to RIG, suggesting that PEG should be considered as the first choice for long-term enteral tube feeding. Further prospective randomized studies are needed to evaluate and compare the safety of these two different methods of gastrostomy.
Gastrostomy feeding is recommended for patients with dysphagia to improve nutrition.1 Previously, gastrostomy tubes were placed solely using the open surgical technique. However, percutaneous endoscopic gastrostomy (PEG) has replaced surgical placement since it was introduced by Gauderer and Ponsky in 1980.2 Radiologically-inserted gastrostomy (RIG) was also introduced in 1981 and has been used as an alternative to PEG for enteral feeding with a high rate of technical success under local anesthesia.3 Although PEG and RIG are both considered minimally invasive, they are associated with considerable early mortality (30-day mortality), which has been reported as 5.8% to 28% for PEG and 1% to 40% for RIG.456789 Furthermore, various complications such as wound infections, hemorrhage, hematoma formation, aspiration pneumonia, or gastric perforation may develop after gastrostomy. Although pneumoperitoneum is a complication which is usually considered minor, a previous study reported mortality related to pneumoperitoneum after PEG insertion.10 Considering that less invasive alternatives do exist, such as a nasogastric tube insertion for short-term tube feeding, it is important to evaluate the safety of gastrostomy. Thirty-day mortality is one of the most important surrogate indices for evaluating the safety of percutaneous gastrostomy.11112 However, no systematic reviews or meta-analyses have compared PEG and RIG in terms of 30-day mortality rates. In the absence of sufficient evidence, the choice between PEG and RIG is largely based on expert opinions or institutional preference. The aim of the present study was to perform a systemic review and meta-analysis comparing the 30-day mortality rates after PEG and RIG.
The protocol for this review was developed prospectively. The checklist and flow chart of the preferred reporting items for systematic review and meta-analyses (PRISMA) statement were used for the review process and data extraction.13 We prepared a prespecified protocol of the specific patients (patients with dysphagia), interventions (PEG), controls (RIG), the primary outcome (the 30-day mortality), the criteria for study selection, and the assessment of study quality.
To identify all available studies, a detailed search was conducted according to the PRISMA guidelines.12 A systematic search was performed in major electronic databases (MEDLINE, Cochrane library, Scopus, and Embase) using the following search terms: "Gastrostomy" AND "Endoscopic" AND "Radiologic*". The search was restricted to literature from January 1980 to May 2015. Non-English or non-Korean language trials were translated and included.
The following selection criteria were applied: (1) patients with dysphagia or in need of prolonged tube feeding; (2) randomized controlled trials (RCT) or other comparative studies comparing 30-day mortality between PEG and RIG; (3) outcome measures of 30-day mortality; and (4) studies that were published in peer-reviewed journals. As there have been only two randomized control trials comparing PEG versus RIG to date, this analysis included both prospective and retrospective comparative studies.
Studies were excluded when they met any of the following criteria: (1) preclinical studies, case reports, case series, or review articles; (2) studies with RIG placement after failure of PEG; (3) studies with no data pertaining to 30-day mortality rates; or (4) studies with no cases of 30-day mortality in either the PEG and the RIG group or duplicated reports from the same patient.
The results of the database search were imported into EndNote X7 software (Thomson Reuters, Philadelphia, PA, USA) and combined into one group to remove duplicated references. Two authors (J.H.L. and S.H.C.) independently assessed the titles and abstracts of all the studies identified by the search. Full texts of potentially eligible articles were obtained and reviewed to assess whether the studies fulfilled the inclusion criteria. Any disagreements between reviewers were resolved through discussion. When an agreement was not achieved, another author (C.L.) was consulted.
Two independent authors (J.H.L. and S.H.C.) analyzed each article and extracted data using a predefined data extraction worksheet. The following information was extracted from each article: title, first author, year of publication, country of origin, study design, patient demographics, the interventions being compared, number of patients, and the 30-day mortality rate.
A meta-analysis was carried out using STATA version 14 (Stata Corp, Texas, TX, USA). We used the STATA metan command for fixed-model (Mantel-Haenszel) analysis and random-effect (DerSimonian and Laird) analysis. We excluded trials with no cases of 30-day mortality in either intervention groups. Differences between the PEG and RIG groups were expressed as the pooled OR with 95% CI. We also used the STATA metan command to create a forest plot and calculate the pooled prevalence. Publication bias was presented graphically by funnel plots of the standard difference in means versus the standard error using the "metafunnel" command and assessed by the Egger's test using the "metabias" command. Subgroup analysis was performed in each group of patients with motor neuron disease and head and neck cancer.
According to the PRISMA flowchart, the selection results are reported in Fig. 1.13 We identified 828 citations through the electronic database search, and 445 citations remained after duplicates were removed. Initial screening of the titles and abstracts resulted in the exclusion of 414 citations, and full texts of the remaining 31 citations were reviewed in detail.891617181920212223242526272829303132333435363738394041424344 An additional 16 citations were excluded after reviewing the full articles, for the following reasons: duplicated reports,424344 noncomparative studies,37 studies with RIG placement after failure of PEG,2535 no data present on 30-day mortality,294041 no reported case of 30-day mortality,192730343638 and review articles.1721 Fifteen published studies including two RCTs1639 and 13 NRCTs891920222324262831323342 were included in the final analysis.
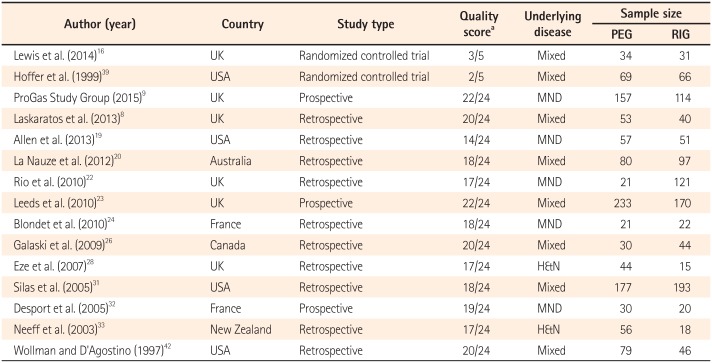
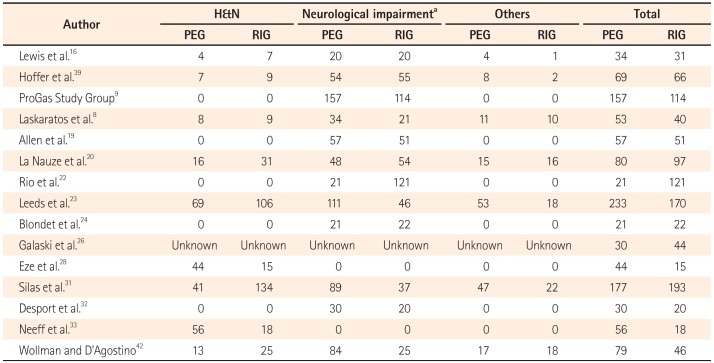
A total of 1,135 patients with PEG insertion and 1,048 patients with RIG insertion were included in the 15 studies. Blinding was not applied to the two RCTs, and the Jadad scores of the two studies were 2 and 3. The quality of the NRCTs was measured by the MINORS score, and the estimated mean MINORS score was 18.6 (95% CI, 17.27-19.95). The characteristics of these studies and quality assessment scores are detailed in Table 1. These studies were published from 1997 to 2015. All of the studies were performed in Western countries (six in the UK, four in the USA, two in France, and one in Australia, Canada, and New Zealand, each). The underlying diseases of the patients varied (mixed in eight studies, motor neuron disease in five studies, head and neck cancer in two studies), and the sample sizes of the studies ranged from 43 to 403 patients. Detailed indications are presented in Table 2.
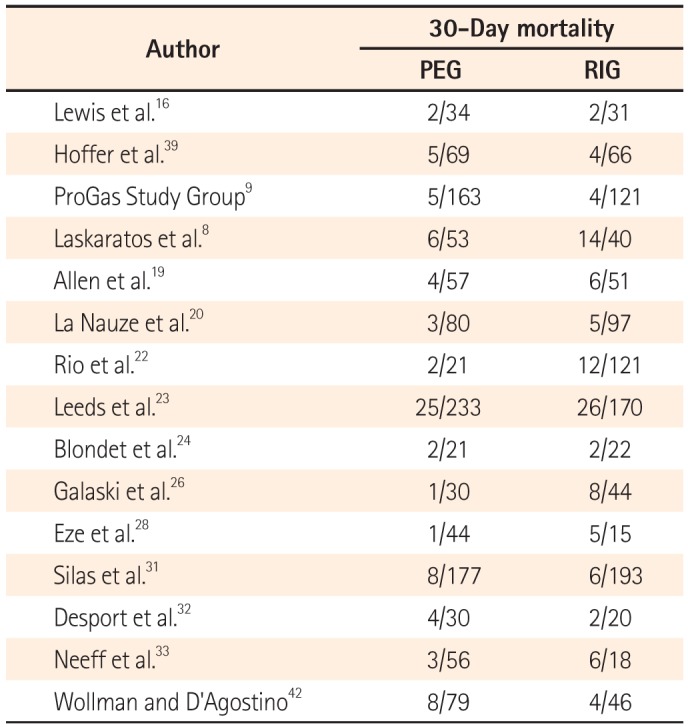
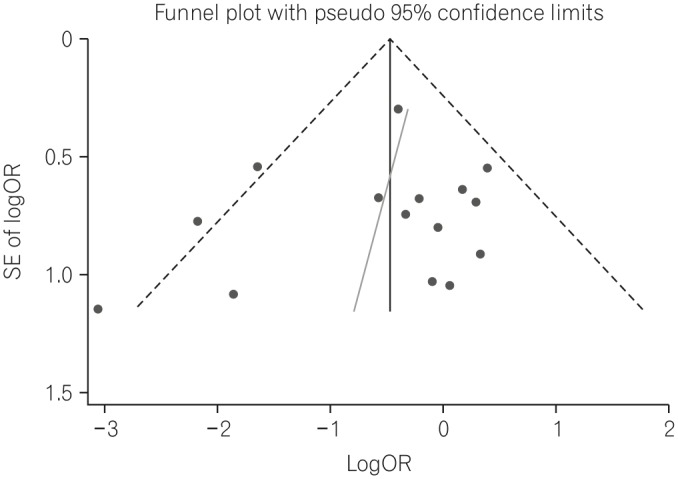
The 30-day mortality rates reported by each study are shown in Table 3 and Fig. 2A. A pooled analysis of the two RCTs with the fixed model showed no difference between the two groups (OR, 1.18; 95% CI, 0.38-3.66; P=0.770). As this result was thought to be due to the small number of RCTs included, we performed a pooled analysis with RCTs and NRCTs combined. The pooled analysis of the ORs demonstrated a statistically significant reduction of 30-day mortality rates in the PEG groups compared with the RIG groups (OR, 0.60; 95% CI, 0.44-0.82; P=0.001) (Fig. 2A) in a fixed effects model with moderate heterogeneity (heterogeneity χ2=22.68, I2=38.7%, P=0.063). Because of this moderate heterogeneity, we next used a random effects model to perform a pooled analysis of the ORs. In the random effects model, the pooled analysis of the ORs also demonstrated a statistically significant reduction of 30-day mortality rates in the PEG groups (OR, 0.60; 95% CI, 0.38-0.94; P=0.026) (Fig. 2B). The funnel plot assay (Fig. 3) and the Egger's test (P=0.542) did not show evidence of publication bias.
Next, we calculated the pooled 30-day mortality rate in the PEG and RIG groups. The pooled prevalence of 30-day mortality of the PEG group was 5.5% (95% CI, 4.0%-6.9%) in a fixed effects model with low heterogeneity (heterogeneity χ2=17.09, I2=18.1%, P=0.251). The pooled prevalence of 30-day mortality of the RIG group was 6.7% (95% CI, 5.1%-8.3%) in a fixed effects model with high heterogeneity (heterogeneity χ2=50.92, I2=72.5%, P<0.001). Because of this high heterogeneity, we then applied a random effects model. In the random effects model, the pooled prevalence of 30-day mortality of the RIG group was 10.5% (95% CI, 6.8%-14.3%).
A total of five studies919222432 were performed in patients with motor neuron disease. Pooled analysis of the ORs showed no significant difference in 30-day mortality between the PEG group and RIG group (OR, 0.84; 95% CI, 0.43-1.69; P=0.637) (Fig. 4A) with low heterogeneity (heterogeneity χ2=0.71, I2<0.1%, P=0.950). The funnel plot assay (Fig. 4B) and the Egger's test (P=0.124) did not show evidence of publication bias.
A total of three studies232833 were performed in patients with head and neck cancer including a subgroup analysis study.23 The pooled OR of 30-day mortality was significantly reduced in the PEG group compared to the RIG group (OR, 0.09; 95% CI, 0.03-0.28; P<0.001) (Fig. 5A) with low heterogeneity (heterogeneity χ2=0.51, I2<0.1%, P=0.775). The funnel plot assay (Fig. 5B) and the Egger's test (P=0.060) did not show evidence of publication bias.
This is the first systematic review and meta-analysis comparing the 30-day mortality rates of PEG versus RIG. We reviewed 30-day mortality after PEG or RIG by summarizing 15 peer-reviewed studies, including 1,135 patients who underwent PEG and 1,048 patients who underwent RIG. A significantly lower risk of 30-day morality was found in the PEG group compared to that in the RIG group (OR, 0.60; 95% CI, 0.38-0.94; P=0.026). This finding supports the current trend of clinical practice, in which PEG is the method of choice for long-term enteral tube feeding, and RIG is an alternative method with a high rate of technical success for those who refuse endoscopy or are unsuitable for PEG.
PEG and RIG placement are generally known as safe and feasible procedures with low mortality rates.9 In this meta-analysis, the pooled 30-day mortality rate of the PEG group was 5.5% (95% 4.0%-6.9%) and that of the RIG group was 10.5% (95% CI, 6.8%-14.3%). These findings suggest that more careful exploration of the risks and benefits of the procedure is needed, especially for RIG, because less invasive methods exist for short-term tube feeding, such as nasogastric tube insertion.
The reason for the lower 30-day mortality rate in the PEG group could be due to the following: first, administration of prophylactic antibiotics is recommended in PEG and is not generally recommended in RIG,45 which may have resulted in a reduction in the rates of peristomal infection and other acute infectious complications in PEG;893133 second, the tubes used in the two methods differ in shape, as the RIG tubes are relatively thinner in diameter (10-14 F) than the PEG tubes (20 F), and clogging of the feeding tube or tube blockage would be more frequent in tubes of smaller diameters; 1 furthermore, as RIG tubes are fixed with a balloonretention system, they are not as securely fixed as PEG tubes, and peristomal leakage or tube displacement would be more frequent.2833 These differences in tubes may have induced a significant increase in the rate of tube related complications.9 Furthermore, there were differences in underlying diseases between the two groups. The PEG group contained more patients with neurological impairment, while the RIG group included more patients with head and neck cancer. These differences might have influenced the outcomes of the study.
In a subgroup analysis of patients with motor neuron disease, the 30-day mortality rates of the PEG and RIG groups were not significantly different. The PEG procedure in patients with motor neuron disease was generally considered more risky due to probable respiratory distress.24 However, our results provide evidence that PEG is not inferior to RIG in these patients.
The subgroup analysis of patients with head and neck cancer demonstrated a statistically significant reduction in the 30-day mortality rate of the PEG group compared to that of RIG group. In general, a radiological approach is preferred in patients with head and neck cancer because of concerns about tumor seeding and technical problems with oral insertion due to the location of the tumor. However, only three studies were conducted in patients with head and neck cancer to compare 30-day mortality in PEG versus RIG; the small study effect demonstrated by the Egger's test was not significant (P=0.06). Considering these results, PEG is thought to be a safe method for gastrostomy even in patients with head and neck cancer.
There are several limitations to this study. First, most studies enrolled in this meta-analysis were observational studies without randomization of insertion methods, and all the groups were not matched exactly. Although gastrostomy has been widely used, there is a lack of randomized trials on this issue. Because of difficult ethical problems surrounding feeding and the issues of vulnerability in patients with impaired neurology, large randomized controlled studies are not easily performed and are less motivating in changing clinical practice.46 Although many researchers argue that combining analyses of RCTs and NRCTs in a meta-analysis should be avoided, both study designs contain strengths and weaknesses in principle. Additionally, including results from observational studies has been suggested as a means of improving the inferences made only by RCTs. We searched peer-reviewed articles only, and excluded biased studies. Therefore, we believe that this meta-analysis including RCTs and NRCTs had the power to provide better inferences on an issue rarely examined in the context of a RCT. Second, we could not collect data on albumin and CRP levels, which are known as predictive factors for 30-day mortality after gastrostomy. 1 Furthermore, information on the use of prophylactic antibiotics could not be collected, as these could have introduced selection bias. However, we enrolled a comparative study with a higher quality to minimize these uninvestigated selection biases.
In conclusion, PEG was associated with a lower probability of 30-day mortality, suggesting that it could become the first choice for long-term enteral tube feeding. Further randomized prospective studies are needed to evaluate and compare the safety of gastrostomy tube insertion by the two different methods investigated in this study.
ACKNOWLEDGEMENT
Seoul National University Hospital Medical Research Collaborating Center (SNUH MRCC) contributed to the statistical analysis in this report.
References
1. Lee C, Im JP, Kim JW, et al. Risk factors for complications and mortality of percutaneous endoscopic gastrostomy: a multicenter, retrospective study. Surg Endosc. 2013; 27:3806–3815. PMID: 23644838.

2. Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980; 15:872–875. PMID: 6780678.

3. Preshaw RM. A percutaneous method for inserting a feeding gastrostomy tube. Surg Gynecol Obstet. 1981; 152:658–660. PMID: 6784260.
4. Sanders DS, Carter MJ, D'Silva J, James G, Bolton RP, Bardhan KD. Survival analysis in percutaneous endoscopic gastrostomy feeding: a worse outcome in patients with dementia. Am J Gastroenterol. 2000; 95:1472–1475. PMID: 10894581.

5. Richter-Schrag HJ, Richter S, Ruthmann O, Olschewski M, Hopt UT, Fischer A. Risk factors and complications following percutaneous endoscopic gastrostomy: a case series of 1041 patients. Can J Gastroenterol. 2011; 25:201–206. PMID: 21523261.

6. Zopf Y, Maiss J, Konturek P, Rabe C, Hahn EG, Schwab D. Predictive factors of mortality after PEG insertion: guidance for clinical practice. JPEN J Parenter Enteral Nutr. 2011; 35:50–55. PMID: 21224433.
7. Lowe AS, Laasch HU, Stephenson S, et al. Multicentre survey of radiologically inserted gastrostomy feeding tube (RIG) in the UK. Clin Radiol. 2012; 67:843–854. PMID: 22682703.

8. Laskaratos FM, Walker M, Walker M, et al. Predictive factors for early mortality after percutaneous endoscopic and radiologically-inserted gastrostomy. Dig Dis Sci. 2013; 58:3558–3565. PMID: 23975341.

9. ProGas Study Group. Gastrostomy in patients with amyotrophic lateral sclerosis (ProGas): a prospective cohort study. Lancet Neurol. 2015; 14:702–709. PMID: 26027943.
10. Park WY, Lee TH, Lee JS, et al. Reappraisal of pneumoperitoneum after percutaneous endoscopic gastrostomy. Intest Res. 2015; 13:313–317. PMID: 26576136.

11. Janes SE, Price CS, Khan S. Percutaneous endoscopic gastrostomy: 30-day mortality trends and risk factors. J Postgrad Med. 2005; 51:23–28. PMID: 15793334.
12. Blomberg J, Lagergren P, Martin L, Mattsson F, Lagergren J. Albumin and C-reactive protein levels predict short-term mortality after percutaneous endoscopic gastrostomy in a prospective cohort study. Gastrointest Endosc. 2011; 73:29–36. PMID: 21074760.

13. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097. DOI: 10.1371/journal.pmed.1000097. PMID: 19621072.

14. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996; 17:1–12. PMID: 8721797.

15. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003; 73:712–716. PMID: 12956787.

16. Lewis S, Jackson S, Latchford A. Randomized study of radiologic vs endoscopic placement of gastrojejunostomies in patients at risk of aspiration pneumonia. Nutr Clin Pract. 2014; 29:498–503. PMID: 24759762.

17. Nugent B, Lewis S, O'Sullivan JM. Enteral feeding methods for nutritional management in patients with head and neck cancers being treated with radiotherapy and/or chemotherapy. Cochrane Database Syst Rev. 2013; (1):CD007904. DOI: 10.1002/14651858.CD007904.pub3. PMID: 23440820.

18. McAllister P, MacIver C, Wales C, et al. Gastrostomy insertion in head and neck cancer patients: a 3 year review of insertion method and complication rates. Br J Oral Maxillofac Surg. 2013; 51:714–718. PMID: 23954134.

19. Allen JA, Chen R, Ajroud-Driss S, et al. Gastrostomy tube placement by endoscopy versus radiologic methods in patients with ALS: a retrospective study of complications and outcome. Amyotroph Lateral Scler Frontotemporal Degener. 2013; 14:308–314. PMID: 23286755.

20. La Nauze RJ, Collins K, Lyon S, et al. Outcomes of percutaneous endoscopic gastrostomy versus radiologically inserted gastrostomy tube insertion at a tertiary hospital. e-SPEN Journal. 2012; 7:e144–e148. DOI: 10.1016/j.clnme.2012.05.001.

21. Burkitt P, Carter LM, Smith AB, Kanatas A. Outcomes of percutaneous endoscopic gastrostomy and radiologically inserted gastrostomy in patients with head and neck cancer: a systematic review. Br J Oral Maxillofac Surg. 2011; 49:516–520. PMID: 20952109.

22. Rio A, Ellis C, Shaw C, et al. Nutritional factors associated with survival following enteral tube feeding in patients with motor neurone disease. J Hum Nutr Diet. 2010; 23:408–415. PMID: 20487174.

23. Leeds JS, McAlindon ME, Grant J, Robson HE, Lee FK, Sanders DS. Survival analysis after gastrostomy: a single-centre, observational study comparing radiological and endoscopic insertion. Eur J Gastroenterol Hepatol. 2010; 22:591–596. PMID: 19966570.

24. Blondet A, Lebigot J, Nicolas G, et al. Radiologic versus endoscopic placement of percutaneous gastrostomy in amyotrophic lateral sclerosis: multivariate analysis of tolerance, efficacy, and survival. J Vasc Interv Radiol. 2010; 21:527–533. PMID: 20172742.

25. Grant DG, Bradley PT, Pothier DD, et al. Complications following gastrostomy tube insertion in patients with head and neck cancer: a prospective multi-institution study, systematic review and meta-analysis. Clin Otolaryngol. 2009; 34:103–112. PMID: 19413607.

26. Galaski A, Peng WW, Ellis M, Darling P, Common A, Tucker E. Gastrostomy tube placement by radiological versus endoscopic methods in an acute care setting: a retrospective review of frequency, indications, complications and outcomes. Can J Gastroenterol. 2009; 23:109–114. PMID: 19214286.

27. MacLean AA, Alvarez NR, Davies JD, Lopez PP, Pizano LR. Complications of percutaneous endoscopic and fluoroscopic gastrostomy tube insertion procedures in 378 patients. Gastroenterol Nurs. 2007; 30:337–341. PMID: 18049202.

28. Eze N, Jefford JM, Wolf D, Williamson P, Neild P. PEG and RIG tube feeding in head and neck patients: a retrospective review of complications and outcome. J Eval Clin Pract. 2007; 13:817–819. PMID: 17824878.

29. Shaw AS, Ampong MA, Rio A, et al. Survival of patients with ALS following institution of enteral feeding is related to preprocedure oximetry: a retrospective review of 98 patients in a single centre. Amyotroph Lateral Scler. 2006; 7:16–21. PMID: 16546754.

30. Rustom IK, Jebreel A, Tayyab M, England RJ, Stafford ND. Percutaneous endoscopic, radiological and surgical gastrostomy tubes: a comparison study in head and neck cancer patients. J Laryngol Otol. 2006; 120:463–466. PMID: 16772054.

31. Silas AM, Pearce LF, Lestina LS, et al. Percutaneous radiologic gastrostomy versus percutaneous endoscopic gastrostomy: a comparison of indications, complications and outcomes in 370 patients. Eur J Radiol. 2005; 56:84–90. PMID: 16168268.

32. Desport JC, Mabrouk T, Bouillet P, Perna A, Preux PM, Couratier P. Complications and survival following radiologically and endoscopically-guided gastrostomy in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005; 6:88–93. PMID: 16036431.

33. Neeff M, Crowder VL, McIvor NP, Chaplin JM, Morton RP. Comparison of the use of endoscopic and radiologic gastrostomy in a single head and neck cancer unit. ANZ J Surg. 2003; 73:590–593. PMID: 12887525.

34. Laasch HU, Wilbraham L, Bullen K, et al. Gastrostomy insertion: comparing the options: PEG, RIG or PIG? Clin Radiol. 2003; 58:398–405. PMID: 12727170.
35. Thornton FJ, Fotheringham T, Alexander M, Hardiman O, McGrath FP, Lee MJ. Amyotrophic lateral sclerosis: enteral nutrition provision: endoscopic or radiologic gastrostomy? Radiology. 2002; 224:713–717. PMID: 12202704.

36. Kohout P, Manák J, Vacek Z, Tucek D. Puncture gastrostomy in oncologic patients. Vnitr Lek. 2002; 48:626–628. PMID: 12197404.
37. Dinkel HP, Beer KT, Zbären P, Triller J. Establishing radiological percutaneous gastrostomy with balloon-retained tubes as an alternative to endoscopic and surgical gastrostomy in patients with tumours of the head and neck or oesophagus. Br J Radiol. 2002; 75:371–377. PMID: 12000697.

38. Bazarah SM, Al-Rawas M, Akbar H, Qari Y. Percutaneous gastrostomy and gastrojejunostomy: radiological and endoscopic approach. Ann Saudi Med. 2002; 22:38–42. PMID: 17259765.

39. Hoffer EK, Cosgrove JM, Levin DQ, Herskowitz MM, Sclafani SJ. Radiologic gastrojejunostomy and percutaneous endoscopic gastrostomy: a prospective, randomized comparison. J Vasc Interv Radiol. 1999; 10:413–420. PMID: 10229468.

40. Cosentini EP, Sautner T, Gnant M, Winkelbauer F, Teleky B, Jakesz R. Outcomes of surgical, percutaneous endoscopic, and percutaneous radiologic gastrostomies. Arch Surg. 1998; 133:1076–1083. PMID: 9790204.

41. Barkmeier JM, Trerotola SO, Wiebke EA, et al. Percutaneous radiologic, surgical endoscopic, and percutaneous endoscopic gastrostomy/gastrojejunostomy: comparative study and cost analysis. Cardiovasc Intervent Radiol. 1998; 21:324–328. PMID: 9688801.

42. Wollman B, D'Agostino HB. Percutaneous radiologic and endoscopic gastrostomy: a 3-year institutional analysis of procedure performance. AJR Am J Roentgenol. 1997; 169:1551–1553. PMID: 9393163.

43. Wollman B, D'agostino HB, Walus-Wigle JR, Easter DW, Beale A. Percutaneous radiologic and endoscopic gastrostomy: a two-year evaluation. Minim Invasive Ther Allied Technol. 1996; 5:544–546.

44. Wollman B, D'Agostino HB, Walus-Wigle JR, Easter DW, Beale A. Radiologic, endoscopic, and surgical gastrostomy: an institutional evaluation and meta-analysis of the literature. Radiology. 1995; 197:699–704. PMID: 7480742.

45. ASGE Standards of Practice Committee. Jain R, Maple JT, et al. The role of endoscopy in enteral feeding. Gastrointest Endosc. 2011; 74:7–12. PMID: 21704804.

46. Kelly AG, Sahin B, Holloway RG. Ethical considerations in stroke patients. Curr Opin Neurol. 2014; 27:61–65. PMID: 24296638.

Fig. 1
Preferred reporting items for systematic review and meta-analyses diagram of the included articles.
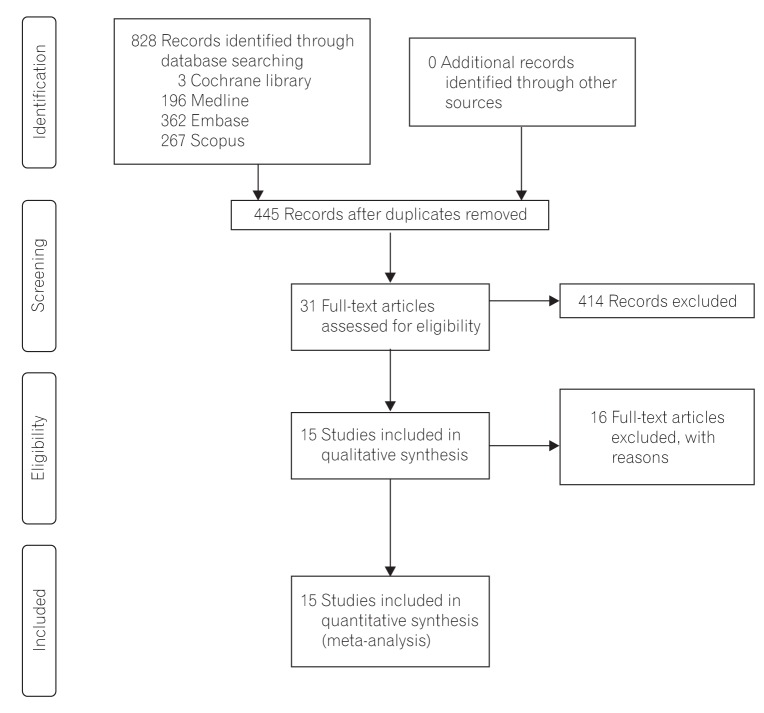
Fig. 2
Forest plot of 30-day mortality after gastrostomy tube insertion. (A) Fixed effect model. (B) Random effect model.
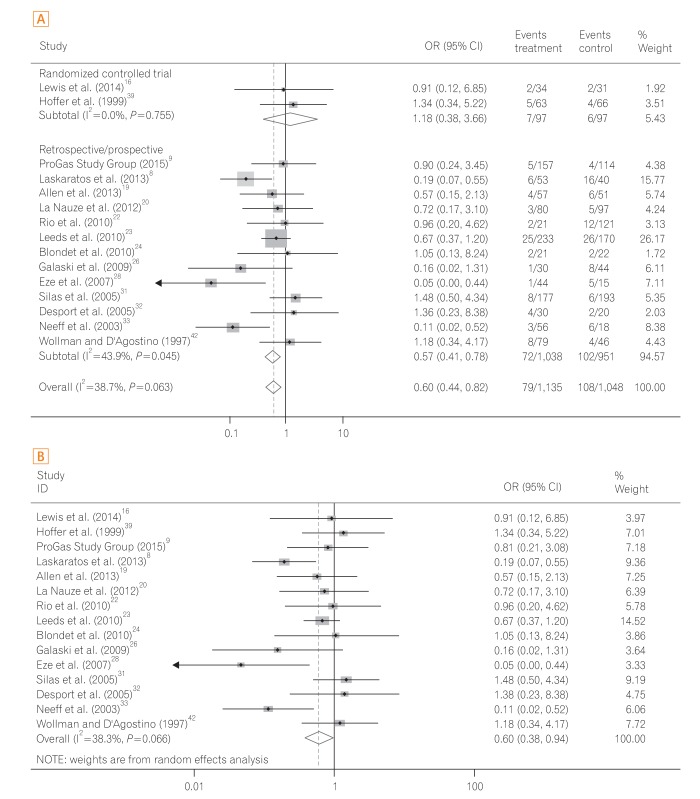
Fig. 4
Subgroup analysis of patients with motor neuron disease. (A) Forest plot of 30-day mortality after gastrostomy tube insertion. (B) Funnel plot of 30-day mortality after gastrostomy tube insertion.
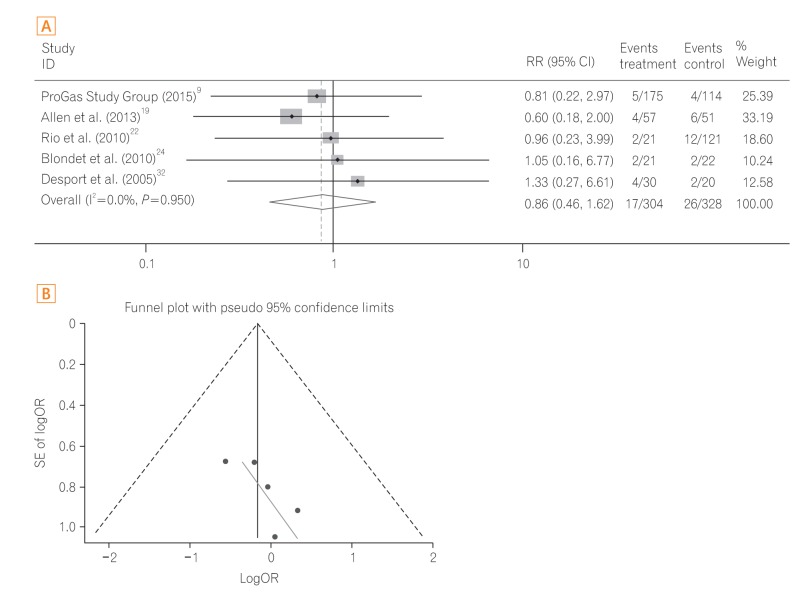
Fig. 5
Subgroup analysis in patients with head and neck cancer. (A) Forest plot of 30-day mortality after gastrostomy tube insertion. (B) Funnel plot of 30-day mortality after gastrostomy tube insertion.
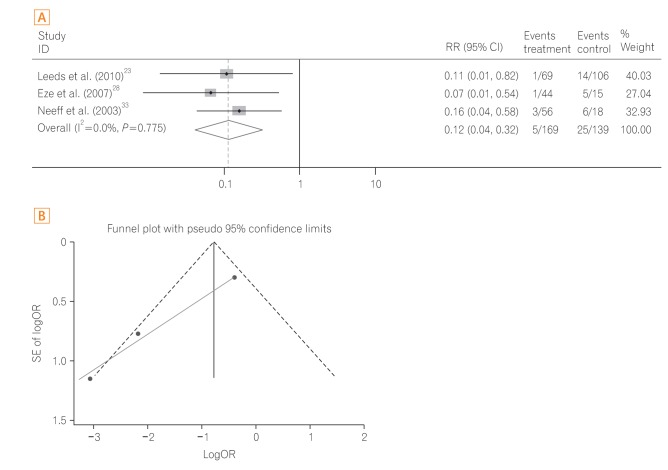
Table 1
Characteristics of Trials Included in the Meta-Analysis
| Author (year) | Country | Study type | Quality scorea | Underlying disease | Sample size | |
|---|---|---|---|---|---|---|
| PEG | RIG | |||||
| Lewis et al. (2014)16 | UK | Randomized controlled trial | 3/5 | Mixed | 34 | 31 |
| Hoffer et al. (1999)39 | USA | Randomized controlled trial | 2/5 | Mixed | 69 | 66 |
| ProGas Study Group (2015)9 | UK | Prospective | 22/24 | MND | 157 | 114 |
| Laskaratos et al. (2013)8 | UK | Retrospective | 20/24 | Mixed | 53 | 40 |
| Allen et al. (2013)19 | USA | Retrospective | 14/24 | MND | 57 | 51 |
| La Nauze et al. (2012)20 | Australia | Retrospective | 18/24 | Mixed | 80 | 97 |
| Rio et al. (2010)22 | UK | Retrospective | 17/24 | MND | 21 | 121 |
| Leeds et al. (2010)23 | UK | Prospective | 22/24 | Mixed | 233 | 170 |
| Blondet et al. (2010)24 | France | Retrospective | 18/24 | MND | 21 | 22 |
| Galaski et al. (2009)26 | Canada | Retrospective | 20/24 | Mixed | 30 | 44 |
| Eze et al. (2007)28 | UK | Retrospective | 17/24 | H&N | 44 | 15 |
| Silas et al. (2005)31 | USA | Retrospective | 18/24 | Mixed | 177 | 193 |
| Desport et al. (2005)32 | France | Prospective | 19/24 | MND | 30 | 20 |
| Neeff et al. (2003)33 | New Zealand | Retrospective | 17/24 | H&N | 56 | 18 |
| Wollman and D'Agostino (1997)42 | USA | Retrospective | 20/24 | Mixed | 79 | 46 |
Table 2
Indications for Gastrostomy
| Author | H&N | Neurological impairmenta | Others | Total | ||||
|---|---|---|---|---|---|---|---|---|
| PEG | RIG | PEG | RIG | PEG | RIG | PEG | RIG | |
| Lewis et al.16 | 4 | 7 | 20 | 20 | 4 | 1 | 34 | 31 |
| Hoffer et al.39 | 7 | 9 | 54 | 55 | 8 | 2 | 69 | 66 |
| ProGas Study Group9 | 0 | 0 | 157 | 114 | 0 | 0 | 157 | 114 |
| Laskaratos et al.8 | 8 | 9 | 34 | 21 | 11 | 10 | 53 | 40 |
| Allen et al.19 | 0 | 0 | 57 | 51 | 0 | 0 | 57 | 51 |
| La Nauze et al.20 | 16 | 31 | 48 | 54 | 15 | 16 | 80 | 97 |
| Rio et al.22 | 0 | 0 | 21 | 121 | 0 | 0 | 21 | 121 |
| Leeds et al.23 | 69 | 106 | 111 | 46 | 53 | 18 | 233 | 170 |
| Blondet et al.24 | 0 | 0 | 21 | 22 | 0 | 0 | 21 | 22 |
| Galaski et al.26 | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | 30 | 44 |
| Eze et al.28 | 44 | 15 | 0 | 0 | 0 | 0 | 44 | 15 |
| Silas et al.31 | 41 | 0134 | 89 | 37 | 47 | 22 | 177 | 193 |
| Desport et al.32 | 0 | 0 | 30 | 20 | 0 | 0 | 30 | 20 |
| Neeff et al.33 | 56 | 18 | 0 | 0 | 0 | 0 | 56 | 18 |
| Wollman and D'Agostino42 | 13 | 25 | 84 | 25 | 17 | 18 | 79 | 46 |
Table 3
Thirty-Day Mortality Rate
| Author | 30-Day mortality | |
|---|---|---|
| PEG | RIG | |
| Lewis et al.16 | 2/34 | 2/31 |
| Hoffer et al.39 | 5/69 | 4/66 |
| ProGas Study Group9 | 5/163 | 4/121 |
| Laskaratos et al.8 | 6/53 | 14/40 |
| Allen et al.19 | 4/57 | 6/51 |
| La Nauze et al.20 | 3/80 | 5/97 |
| Rio et al.22 | 2/21 | 12/121 |
| Leeds et al.23 | 25/233 | 26/170 |
| Blondet et al.24 | 2/21 | 2/22 |
| Galaski et al.26 | 1/30 | 8/44 |
| Eze et al.28 | 1/44 | 5/15 |
| Silas et al.31 | 8/177 | 6/193 |
| Desport et al.32 | 4/30 | 2/20 |
| Neeff et al.33 | 3/56 | 6/18 |
| Wollman and D'Agostino42 | 8/79 | 4/46 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download