Abstract
Coronavirus disease 2019 (COVID-19) increases the risk of mortality and hospitalization in immunocompromised patients, including kidney transplant recipients (KTRs) receiving immunosuppressants. Several vaccines for COVID-19 have been developed and proven effective in decreasing the incidence of COVID-19 and the rate of progression to severe COVID-19. However, breakthrough infections have also been reported in vaccinated patients. We report cases from our center of delayed exacerbated pneumonia from COVID-19 in vaccinated KTRs receiving immunosuppressants. Of the 900 KTRs who had been vaccinated for COVID-19 and were followed up at our center from January 1, 2022, to April 30, 2022 (during the Omicron variant outbreak), 126 contracted COVID-19 (incidence rate, 14%). Thirty-four (27%) in this group were hospitalized due to COVID-19. Twenty patients did not have pneumonia but had symptoms of upper respiratory tract infection or diarrhea, which improved with conservative treatment. Nine of the 14 patients with pneumonia had delayed onset or exacerbated pneumonia 1 week after their COVID-19 diagnosis. They were treated with remdesivir, and most recovered. One patient died due to progressive pneumonia and pneumothorax. It is important that KTRs who are taking immunosuppressants be observed closely and for a prolonged period after a COVID-19 diagnosis, irrespective of their COVID-19 vaccination status.
The World Health Organization declared that coronavirus disease 2019 (COVID-19) was a global pandemic on March 11, 2020. Reports on the prevalence, incidence, hospitalization, and mortality rates of COVID-19 have varied worldwide [1]. Providence St. Joseph Health, one of the largest health-care organizations in the United States, reported an 8.7% prevalence of COVID-19 in its facilities between March 1, 2020, and December 31, 2020, with 15.6% of cases requiring hospitalization [1]. Mortality rates worldwide have ranged from 0.9% to 14.3%, depending on the country or region [2]. The clinical features of COVID-19 are diverse, ranging from minor symptoms to death, after an incubation period of 5–14 days [1].
According to a recent meta-analysis, severe COVID-19 was associated with older age and underlying comorbidities, such as hypertension, diabetes, heart disease, and chronic obstructive pulmonary disease [3]. Notably, older age and underlying comorbidities were independently associated with mortality in patients undergoing solid organ transplantation [4]. Korean surveillance data showed that COVID-19 posed a higher risk of fatality in solid organ transplant recipients than in the general population aged 50–79 years [5]. Moreover, mortality due to COVID-19 was reported to be higher in kidney transplant recipients (KTRs, 20%–28%) than in non-KTRs (1%–5%) [6]. According to a sub-registry task force of the Asian Organ Transplantation Registry, the overall mortality rate in Asian KTRs was 23% [7]. In contrast, a comparative study showed that mortality, intensive care unit admission, and mechanical ventilation were not different for KTRs with chronic immunosuppression than for the general population [8]. Therefore, the clinical features and outcomes of COVID-19 in KTRs remain unclear.
Vaccines have been developed to decrease the COVID-19 burden, and governments and societies have encouraged the public to accept immunization when appropriate [9]. In Korea, the rate of vaccination exceeded 80% of the population in December 2021. The Korean government and the Ministry of Health and Welfare required patients with COVID-19 to be quarantined for 7 days to prevent its spread. We report the clinical presentation associated with delayed exacerbated pneumonia due to COVID-19 in vaccinated KTRs who were on immunosuppressants.
This study was approved by the Institutional Review Board of Bong Seng Memorial Hospital (IRB No. BSIRB-2022-008), which waived the requirement for informed consent due to the retrospective nature of this study.
We retrospectively reviewed the medical records of 900 vaccinated KTRs who were followed up between January 1, 2022, and April 30, 2022. This period was the peak of the Omicron variant outbreak in South Korea. Overall, 126 patients with clinical symptoms were diagnosed with COVID-19 (incidence rate, 14%) during this period by a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) real-time polymerase chain reaction (RT-PCR) test after a nasopharyngeal swab. The median age of patients with COVID-19 was 59 years (range, 26–79 years), and 61 patients (48.4%) were men. Thirty-four KTRs (27%) were hospitalized with COVID-19, of whom 31 contracted COVID-19 outside of the hospital and three had hospital-acquired COVID-19. The 20 patients without pneumonia had various symptoms, including general weakness, myalgia, mild fever, sore throat, cough, sputum, and diarrhea, and all improved with conservative therapy. Fourteen patients had pneumonia, and nine of them were hospitalized for more than 1 week after being diagnosed with COVID-19 because of the late onset or exacerbation of their pneumonia (Fig. 1).
The characteristics of the nine patients are summarized in Tables 1 and 2. When initially diagnosed with COVID-19, four patients had no symptoms except myalgia, whereas five patients had symptoms of mild upper respiratory infection, including sore throat, cough, sputum, and myalgia, with or without mild fever or mild diarrhea. Three (33.3%) of the nine patients were men, and the median age was 65 years (range, 41–71 years). The median time since kidney transplantation was 12 years (range, 0.3–28 years). Seven patients had type 2 diabetes and hypertension, and two patients had autosomal dominant polycystic kidney disease. All patients received low-dose steroid therapy and immunosuppressants, including tacrolimus, mycophenolate mofetil (MMF), or mizoribine (Table 1). They had respiratory symptoms, such as cough, sputum, sore throat, myalgia, and fever. One patient (patient 6) had received Paxlovid at the time of his COVID-19 diagnosis. The median time from COVID-19 diagnosis to admission for exacerbated symptoms was 14 days (range, 7–25 days). On admission, the patients were retested for SARS-CoV-2 by RT-PCR, and all tested positive. The median cycle threshold of SARS-CoV-2 by RT-PCR was 21.48 (range, 19.26–23.64). Chest computed tomography showed diffuse ground-glass opacity that was peripherally distributed, and one patient had widespread alveolar consolidation (Table 1). MMF was discontinued in all patients diagnosed with viral pneumonia caused by COVID-19. Remdesivir with methylprednisolone (32 mg) was initiated intravenously, with a 200-mg loading dose on day 1, followed by 100 mg daily for up to 4–9 additional days. The mean duration of hospitalization was 10 days (5–25 days). Two patients (patients 8 and 9) required continuous renal replacement therapy for acute kidney injury. Renal biopsy to differentiate acute rejection could not be performed because one patient (patient 8) strongly declined the procedure since renal replacement therapy had already been under consideration, whereas the other patient (patient 9) had severe pneumonia with invasive mechanical ventilator management and died due to progressive pneumonia and pneumothorax. The renal function of patient 8 recovered after continuous renal replacement therapy for 3 days with a decrease in his serum creatinine level from 5.4 mg/dL to 3.1 mg/dL and a 24-hour urine output >1 L. All patients recovered from pneumonia except for patient 9 (Table 2).
We report on vaccinated KTRs receiving immunosuppressants who had a delayed exacerbation or onset of pneumonia more than 1 week after a COVID-19 diagnosis with mild symptoms. Most patients recovered from the pneumonia with antiviral therapy, but acute kidney injury developed in two cases and one patient died.
A recent single-center trial reported that the rate of positive SARS-CoV-2 by RT-PCR (nasopharyngeal or oropharyngeal swabs), hospitalization, and mortality in KTRs was 28.3%, 44.4%, and 8.9%, respectively [10]. This high mortality rate in KTRs may have been influenced by non-vaccination, although the rate of hospitalization was like that found in our study. The delay in pneumonia infiltration reported in KTRs may be associated with MMF and steroid use [10]. In the general population, although late-onset or persistent complications of COVID-19 have been reported, including active shortness of breath, cough, asthma, and other respiratory complications, no cases of late-onset pneumonia were seen, unlike our study [11]. Another study reported that vaccination could reduce residual symptoms following COVID-19 in the general population [12]. However, these persistent or residual symptoms in the general population are different and less severe than the late-onset and exacerbated symptoms of the KTRs in our study, which required medical attention and subsequent hospitalization. The risk factors and mechanisms that delayed the onset of pneumonia or exacerbated the symptoms in KTRs remain uncertain [10].
In COVID-19, the viral load of SARS-CoV-2 is highest in the respiratory tract at the time of diagnosis, and subsequent viral shedding can continue in the respiratory tract for 14 days [13,14]. During this period, the number of CD8 T cells increases. They mainly eliminate SARS-CoV-2 and mediate viral clearance in the infected respiratory tract by utilizing a variety of effector mechanisms to induce the apoptosis of virus-infected cells [14]. Immunosuppressive agents, such as tacrolimus and MMF, are known to interfere with the proliferation and survival of primary T cells, particularly the generation of memory CD8 T cells [15]. These immunosuppressant effects may be related to the late onset and exacerbated symptoms of pneumonia found in vaccinated KTRs.
In this study, the clinical phenotype of late-onset or exacerbated pneumonia 1 week after a COVID-19 diagnosis was found in vaccinated KTRs who were receiving immunosuppressants, which can delay the onset of pneumonia. Therefore, it is important that KTRs on immunosuppressants be closely observed for a prolonged period after a COVID-19 diagnosis, even when they have been vaccinated against COVID-19. Further studies are needed to compare the clinical features and prognostic precursors of these patients.
REFERENCES
1. Dai CL, Kornilov SA, Roper RT, Cohen-Cline H, Jade K, Smith B, et al. 2021; Characteristics and factors associated with coronavirus disease 2019 infection, hospitalization, and mortality across race and ethnicity. Clin Infect Dis. 73:2193–204. DOI: 10.1093/cid/ciab154. PMID: 33608710. PMCID: PMC7929051.
2. Li J, Huang DQ, Zou B, Yang H, Hui WZ, Rui F, et al. 2021; Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 93:1449–58. DOI: 10.1002/jmv.26424. PMID: 32790106. PMCID: PMC7436673.

3. Zhang YJ, Sun XF, Xie B, Feng WJ, Han SL. 2021; Exploration of severe Covid-19 associated risk factor in China: meta-analysis of current evidence. Int J Clin Pract. 75:e14900. DOI: 10.1111/ijcp.14900. PMCID: PMC8646532.

4. Kates OS, Haydel BM, Florman SS, Rana MM, Chaudhry ZS, Ramesh MS, et al. 2021; Coronavirus disease 2019 in solid organ transplant: a multicenter cohort study. Clin Infect Dis. 73:e4090–9.
5. Kang JM, Kim YJ, Huh K, Kim JM, Park WB, Ahn HJ, et al. 2022; COVID-19 among solid organ transplant recipients in Korea: surveillance data of the Korean Transplantation Society, January 2020 to March 2022. Korean J Transplant. 36:159–63. DOI: 10.4285/kjt.22.0025. PMID: 35919200. PMCID: PMC9296979.
6. Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, et al. 2020; Covid-19 and kidney transplantation. N Engl J Med. 382:2475–7. DOI: 10.1056/NEJMc2011117. PMID: 32329975. PMCID: PMC7200055.
7. Kee T, Jeong JC, Ur-Rashid H, Begum NA, Arakama MH, Danguilan R, et al. 2021; Clinical characteristics, outcomes, and management of COVID-19 in kidney transplant recipients across Asia: an ASTREGO report. Korean J Transplant. 35:218–29. DOI: 10.4285/kjt.21.0024. PMID: 35769859. PMCID: PMC9235460.

8. Monfared A, Akhondzadeh L, Mousazadeh M, Jafari A, Khosravi M, Lebadi M, et al. 2021; COVID-19 in renal transplant recipients and general population: a comparative study of clinical, laboratory, and radiological features, severity, and outcome. Virol J. 18:243. DOI: 10.1186/s12985-021-01713-x. PMID: 34876176. PMCID: PMC8649678.
9. Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, et al. 2021; A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 27:225–8. DOI: 10.1038/s41591-020-1124-9. PMID: 33082575. PMCID: PMC7573523.
10. Asghar MS, Khan MT, Hamid RB, Lal N, Ahmed I, Hameed B, et al. 2021; Clinical characteristics, biochemical markers, and outcomes of postrenal transplant patients with coronavirus diseases 2019: a single-center experience. Saudi J Kidney Dis Transpl. 32:1689–99. DOI: 10.4103/1319-2442.352430. PMID: 35946282.
11. Saberian P, Pazooki B, Hasani-Sharamin P, Garjani K, Ahmadi Hatam Z, Dadashi F, et al. 2022; Persistent/late-onset complications of COVID-19 in general population: a cross-sectional study in Tehran, Iran. Int J Community Based Nurs Midwifery. 10:234–45.
12. Herman B, Wong MC, Viwattanakulvanid P. 2022; Vaccination status, favipiravir, and micronutrient supplementation roles in post-COVID symptoms: a longitudinal study. PLoS One. 17:e0271385. DOI: 10.1371/journal.pone.0271385. PMID: 35862378. PMCID: PMC9302723.

13. Ayhancı T, Toptan H, Özer YE, Uzar S, Enül H, Adıay C, et al. 2022; Shedding of SARS-CoV-2 virus in COVID-19 patients and neutralizing antibody level. Mikrobiyol Bul. 56:416–31. DOI: 10.5578/mb.20229704. PMID: 35960235.
14. Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, Dhariwal J, et al. 2015; RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat Commun. 6:10224. DOI: 10.1038/ncomms10224. PMID: 26687547. PMCID: PMC4703893.
15. Quéméneur L, Beloeil L, Michallet MC, Angelov G, Tomkowiak M, Revillard JP, et al. 2004; Restriction of de novo nucleotide biosynthesis interferes with clonal expansion and differentiation into effector and memory CD8 T cells. J Immunol. 173:4945–52. DOI: 10.4049/jimmunol.173.8.4945. PMID: 15470036.

Fig. 1
Flowchart for identification of the study cohort. COVID-19, coronavirus disease 2019; KTR, kidney transplantation recipient.
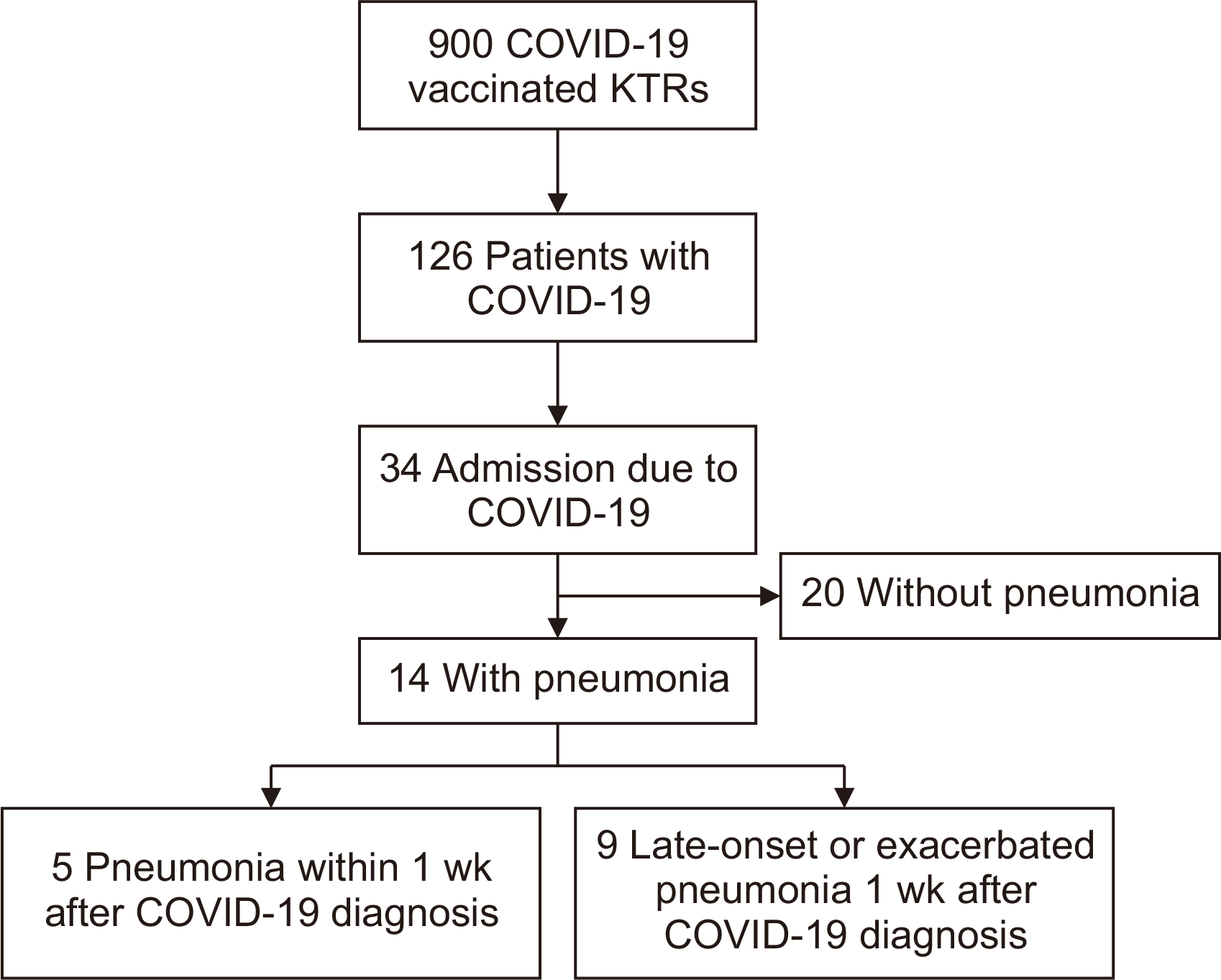
Table 1
Demographics and admission characteristics of KTRs with exacerbated pneumonia 1 week after COVID-19 diagnosis (n=9)
| Variable | Patient no. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Sex/age (yr) | M/65 | F/63 | F/67 | M/64 | F/63 | F/67 | F/41 | M/71 | F/66 |
| Time since transplant (yr) | 28 | 12 | 2 | 0.3 | 10 | 20 | 14 | 3 | 15 |
| Comorbidity | HT, T2DM | HT, T2DM | ADPKD | HT, T2DM | HT, T2DM | HT, T2DM | ADPKD | HT, T2DM | HT, T2DM |
| Immunosuppressant | TAC-MMF | TAC-MMF | TAC-MMF | TAC-MMF | TAC-MMF | TAC-MMF | TAC-MMF | TAC-MMF | TAC-Miz |
| Daily dose of MMF (mg) | 1,000 | 1,500 | 1,500 | 1,500 | 1,500 | 1,000 | 1,000 | 1,500 | - |
| Daily prednisolone (mg) | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Vaccination statusa) | P-P-N | P-P-P | A-A-P | A-A | A-A-M | A-A-A | A-A-P | P-P-P | A-A |
| Last vaccination date | Jan 2022 | Dec 2021 | Dec 2021 | Aug 2021 | Mar 2022 | Dec 2021 | Jan 2022 | Dec 2021 | Aug 2021 |
| Date of COVID-19 | Feb 22, 2022 | Mar 2, 2022 | Mar 2, 2022 | Mar 6, 2022 | Apr 4, 2022 | Mar 5, 2022 | Mar 15, 2022 | Mar 19, 2022 | Feb 26, 2022 |
| Admission characteristic | |||||||||
| RT-PCR Ct for COVID-19 | 21.58 | 23.64 | 23.64 | 19.26 | 21.54 | 19.28 | 21.03 | 21.48 | 21.25 |
| Chest CT finding | Peripheral GGO | Peripheral GGO | Peripheral GGO | Peripheral GGO | Peripheral GGO | Peripheral GGO | Peripheral GGO | Peripheral GGO | Alveolar consolidationb) |
| Laboratory finding | |||||||||
| Serum creatinine (mg/dL) | 1.1 | 0.9 | 0.8 | 1.3 | 1.4 | 1.2 | 0.6 | 5.4 | 4.0 |
| Serum CRP (mg/dL) | 5.02 | 1.81 | 1.25 | 10.96 | 8.25 | 16.21 | 9.81 | 4.17 | 15.92 |
| Hemoglobin (g/dL) | 12.9 | 12.8 | 11.8 | 10 | 8.2 | 10.6 | 9.3 | 8.5 | 11 |
| WBC count (/μL) | 6,200 | 4,600 | 2,300 | 7,000 | 4,600 | 3,600 | 6,600 | 6,300 | 4,600 |
| Platelet count (×103/μL) | 152 | 125 | 129 | 182 | 166 | 360 | 201 | 114 | 120 |
| TAC levels (ng/mL) | 3.8 | 4.2 | 5.3 | 8.7 | 4.5 | 4.8 | 9.2 | 8.2 | 5.7 |
KTR, kidney transplantation recipient; COVID-19, coronavirus diseases 2019; HT, hypertension; T2DM, type 2 diabetes mellitus; ADPKD, autosomal dominant polycystic kidney disease; TAC, tacrolimus; MMF, mycophenolate mofetil; Miz, mizoribine; RT-PCR, real-time polymerase chain reaction; Ct, cycle threshold; CT, computed tomography; GGO, ground-glass opacity; CRP, C-reactive protein; WBC, white blood cell count.
Table 2
Hospital course and outcomes of KTRs with exacerbated pneumonia 1 week after COVID-19 diagnosis (n=9)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download