Abstract
Systemic Epstein-Barr virus (EBV)–positive T-cell lymphoma of childhood (SETLC) is a rare, rapidly progressive, and often fatal disease of children and young adults characterized by monoclonal expansion of EBV-positive T cells in tissues or peripheral blood following infection with EBV. Its distinction from other EBV-positive T-cell lymphoproliferative disorders with overlapping features can be difficult, and particular diagnostic features may not be manifest until autopsy examination. We present the case of a 10-year-old boy with significant disability due to remote traumatic brain injury following non-accidental head trauma who died unexpectedly at home. Given the history of physical abuse and the potential for homicide charges, significant medicolegal implications arose with this case. Pathologic investigation ultimately revealed conclusive diagnostic features of SETLC including extensive proliferation of EBV-positive T cells in multiple organs. A natural manner of death was confirmed, thereby excluding delayed homicide related to complications of non-accidental head trauma.
Systemic Epstein-Barr virus (EBV)-positive T-cell lymphoma of childhood (SETLC) is a rare, rapidly progressive, and often fatal disease of children and young adults characterized by monoclonal expansion of EBV-positive T cells in tissues or peripheral blood following infection with EBV [1]. The disease is most prevalent in eastern Asia and has also been reported in Latin America, but it is relatively rare in western countries [2]. Patients initially experience symptoms of acute viral illness including fever and malaise with subsequent development of hepatosplenomegaly, liver failure, coagulopathy, and pancytopenia. The disease is commonly associated with hemophagocytic syndrome and leads to organ failure, sepsis, and death within days to weeks. Due to its aggressive nature, prompt diagnosis is imperative though often difficult to establish, especially given overlapping features with other EBV-associated T-cell lymphoproliferative disorders [3,4].
Given the often fatal course of SETLC, particular diagnostic features may not be manifest until autopsy examination, making consideration of this entity important in the post-mortem setting [4–7]. Consideration of such rapidly progressive, fatal diseases becomes particularly important in forensic cases with unique medicolegal connotations. We present the case of a 10-year-old boy with significant disability due to remote traumatic brain injury (TBI) following non-accidental head trauma who died unexpectedly at home. Due to his debilitated state, he experienced frequent aspiration pneumonia and reportedly exhibited respiratory distress in the weeks and days prior to his death. Given the history of physical abuse and the potential for homicide charges, significant medicolegal implications arose with this case and magnified the importance of accurately ascertaining the cause and manner of death.
The decedent was a 10-year-old white male who at approximately 20 months of age suffered TBI, multiple skeletal fractures, and bilateral retinal hemorrhages following non-accidental head trauma. Sequelae of the TBI included severe spastic quadriplegia and cognitive impairment in addition to multiple hospital admissions for acute hypoxemic respiratory failure and pneumonia related to aspiration and viral infection. He was discharged home from the most recent admission approximately 5 weeks prior to his death. While aspiration and parainfluenza infection were noted during this admission, no diagnostic testing for EBV was performed, nor was there any documentation of prior EBV infection in the medical record. On the day of his death, the decedent reportedly experienced dyspnea before becoming unresponsive. Despite cardiopulmonary resuscitative efforts, he was ultimately pronounced dead at his residence.
Given the unexpected nature of his death and the medicolegal connotations associated with the history of physical abuse, a forensic autopsy was performed to evaluate the cause and manner of death. Internal gross examination revealed cavitary lesions of the right temporal lobe and the left frontal lobe of the brain consistent with remote blunt head trauma. The lungs displayed diffuse consolidation and parenchymal nodularity imparting a “cobblestone” appearance. Marked, extensive pulmonary hilar and mediastinal lymphadenopathy was identified with the largest lymph node measuring 3.5 cm (Fig. 1). Additionally, the gastroesophageal junction showed prominent mucosal nodularity. No significant hepatosplenomegaly was observed.
Hematoxylin and eosin stained tissue sections were prepared for microscopic examination. A section of pulmonary hilar lymph node showed vague preservation of B-cell follicles and profound paracortical expansion. The expanded paracortex was comprised of a spectrum of lymphocytes, ranging from small forms to more atypical intermediate/large forms with irregular nuclear contours, dispersed chromatin, and prominent nucleoli. Histologic sections of the lungs revealed effacement of the pulmonary architecture by sheets and expanded nodules of lymphoid cells with morphology identical to that in the lymph node along with frequent mitotic figures. Immunohistochemistry (IHC) was applied and showed the majority of atypical lymphocytes to be CD4-positive T cells that were also positive for CD2 and CD3 and showed partial, aberrant loss of CD5 and CD7. The atypical cells were negative for CD56 and CD138. A minority of cells were positive for CD8. In-situ hybridization for EBV encoded RNA (EBER ISH) was performed and showed extensive positivity within atypical lymphoid cells in the lymph node and the lungs (Fig. 2). A section of liver revealed multiple periportal lymphoid aggregates composed predominantly of atypical T cells, while the spleen also demonstrated moderate infiltration of the red pulp by atypical T cells. A diffuse T cell infiltrate with similar morphology was also observed in the stomach disrupting the mucosal architecture. EBER ISH was positive within the atypical lymphocytes in the spleen, the liver, and the gastric mucosa. IHC performed on bone marrow showed an abundance of CD163-positive histiocytes displaying readily observable hemophagocytosis and occasional small, T-cell predominant lymphoid aggregates (Fig. 3).
The gross and microscopic features were consistent with an aggressive, EBV-positive T-cell lymphoma involving multiple organs. Given the fulminant disease onset and rapid, unexpected demise of this pediatric patient, a diagnosis of SETLC was rendered.
EBV-associated lymphoproliferative diseases comprise a wide spectrum of reactive and neoplastic processes that can result in the transformation and proliferation of B, T, or natural killer (NK) cells [8]. Amongst the entities affecting T cells and NK cells specifically, disease features can overlap causing difficulty in establishing a diagnosis. To achieve an accurate diagnosis, a combination of clinical and pathologic details must be considered as key differences exist between the morphologic and temporal aspects of these processes [4,7].
In our case, several aspects aligned with a diagnosis of SETLC. From a temporal standpoint, the rapid demise of the patient was consistent with the fulminant course associated with this entity. Gross and microscopic examination revealed overt T-cell lymphoma (features previously described above) with infiltration of multiple organs, demonstrating the aggressive and systemic nature of this disease. EBER ISH was positive in the T-cell infiltrates confirming an EBV-driven etiology. Additionally, prominent hemophagocytosis was identified in the bone marrow, a finding that is often associated with SETLC [9].
Other EBV-positive T-cell and NK-cell lymphoproliferative disorders to consider along with SETLC include EBV-positive hemophagocytic lymphohistiocytosis (HLH), systemic chronic active EBV infection (CAEBVI), hydroa vacciniforme-like lymphoproliferative disorder, and severe mosquito bite allergy. In our case, hydroa vacciniforme-like lymphoproliferative disorder and severe mosquito bite allergy could be excluded as these are primarily cutaneous disorders without profound systemic manifestations. EBV-positive HLH can present with clinical features similar to those of SETLC including fever, splenomegaly, and pancytopenia. However, while hemophagocytosis can be seen in the bone marrow, the spleen, or the lymph nodes, the proliferation of EBV-positive T cells is relatively small. Systemic CAEBVI can show some morphologic overlap with SETLC including paracortical expansion of lymph nodes, infiltration of multiple organs by EBV-positive T cells, and occasional hemophagocytosis. However, systemic CAEBVI displays reactive, nonspecific inflammatory changes and only subtle lymphoid infiltrates without cytologic atypia, as opposed to SETLC which is marked by neoplastic features, such as prominent lymphocytic proliferation and cytologic atypia. Additionally, systemic CAEBVI follows a more prolonged and less fulminant clinical course than SETLC with infectious symptoms persisting for greater than 3 months [1,9].
Regarding immunophenotype, the neoplastic T cells in our case showed aberrant, partial loss of CD5 and CD7 expression by IHC. Such aberrant loss of pan T-cell antigens is a well-known feature of T-cell lymphomas in general and has been previously described in cases of SETLC specifically [4]. Cases of SETLC occurring after acute EBV infection generally show T cells with a cytotoxic CD8-positive immunophenotype, while those developing from systemic CAEBVI usually display T cells that are CD4-positive. Rarely, cases occur which exhibit both CD4-positive and CD8-positive EBV-infected T cells [2,10]. The majority of neoplastic T cells in our case were CD4-positive, but there was no evidence to suggest progression from previous systemic CAEBVI. Rather, it seems more likely that our case of SETLC is part of the unique minority that displays CD4-positive T cells following acute EBV infection.
Investigation of pediatric deaths can be a challenging aspect of forensic pathology and requires meticulous evaluation of all case aspects, particularly when there is a component of abuse or neglect [11]. This becomes particularly important when evaluating for delayed homicides which can result from complications of a remote injury inflicted by another individual. In such cases, it is crucial to determine not only the immediate cause of death but also the proximate cause of death, which is the original injury without which the fatality would not have occurred. Infection is an immediate cause of death that can be associated with remote blunt force injuries and quadriplegia [12]. In this case, it was necessary to exclude bronchopneumonia as an immediate cause of death, especially given the reported history of dyspnea and recurrent aspiration. Since pathologic investigation revealed conclusive diagnostic features of SETLC, and excluded an etiology related to complications of non-accidental head trauma, the manner of death was determined to be natural rather than homicide.
In conclusion, we present the case of a 10-year-old boy with rapid and unexpected death due to SETLC that was diagnosed at autopsy. Our case is especially informative as it illustrates diagnostic features of SETLC that separate it from other EBV-positive lymphoproliferative disorders of T and NK cells. Additionally, it demonstrates the necessity of thorough forensic examination in the evaluation of potential homicide deaths. Finally, this case highlights the importance of considering aggressive, fulminant T-cell lymphomas as unexpected causes of death, particularly in patients with rapid deterioration and vague, nonspecific clinical presentation [5,6,13].
Notes
Ethics Statement
Given the forensic nature of this case, it did not qualify as human subject research per the Health Sciences Institutional Review Board at the University of Wisconsin-Madison, and prior approval was therefore not required. Appropriate consent was obtained from the referring medical examiner’s office prior to performance of the forensic autopsy.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
References
1. Kim WY, Montes-Mojarro IA, Fend F, Quintanilla-Martinez L. Epstein-Barr virus-associated T and NK-cell lymphoproliferative diseases. Front Pediatr. 2019; 7:71.
2. Quintanilla-Martinez L, Kumar S, Fend F, et al. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. 2000; 96:443–51.
3. Tabanelli V, Agostinelli C, Sabattini E, et al. Systemic Epstein-Barr-virus-positive T cell lymphoproliferative childhood disease in a 22-year-old Caucasian man: a case report and review of the literature. J Med Case Rep. 2011; 5:218.
4. Coffey AM, Lewis A, Marcogliese AN, et al. A clinicopathologic study of the spectrum of systemic forms of EBV-associated T-cell lymphoproliferative disorders of childhood: a single tertiary care pediatric institution experience in North America. Pediatr Blood Cancer. 2019; 66:e27798.
5. Kai K, Koga F, Araki N, et al. Autopsy case of systemic EBV-positive T-cell lymphoma of childhood with marked hepatomegaly in a middle-aged man. Pathol Int. 2017; 67:431–3.
6. Keow JY, Stecho WM, Haig AR, Sangle NA. EBV-positive T/NK-associated lymphoproliferative disorders of childhood: a complete autopsy report. Indian J Pathol Microbiol. 2020; 63:78–82.
7. Wang Z, Kimura S, Iwasaki H, et al. Clinicopathological findings of systemic Epstein-Barr virus-positive T-lymphoproliferative diseases in younger and older adults. Diagn Pathol. 2021; 16:48.
8. Kimura H, Ito Y, Kawabe S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012; 119:673–86.
9. Quintanilla-Martinez L, Ko YH, Kimura H, Jaffe ES. EBV-positive T-cell and NK-cell lymphoproliferative disease of childhood. Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon: IARC;2017. p. 355–63.
10. Su IJ, Chen RL, Lin DT, Lin KS, Chen CC. Epstein-Barr virus (EBV) infects T lymphocytes in childhood EBV-associated hemophagocytic syndrome in Taiwan. Am J Pathol. 1994; 144:1219–25.
11. Knight LD, Collins KA. A 25-year retrospective review of deaths due to pediatric neglect. Am J Forensic Med Pathol. 2005; 26:221–8.
12. Lin P, Gill JR. Delayed homicides and the proximate cause. Am J Forensic Med Pathol. 2009; 30:354–7.
13. Alkhasawneh A, Mubeen A, Gopinath A. Lymphoma in autopsy cases. Forensic Sci Med Pathol. 2018; 14:327–31.
Fig. 1
Gross findings at autopsy. (A) Cavitary lesion in the right temporal lobe (arrow) consistent with remote blunt head trauma. (B) Pulmonary parenchyma with diffuse nodularity imparting a “cobblestone” appearance. (C) Markedly prominent pulmonary hilar and mediastinal lymphadenopathy.
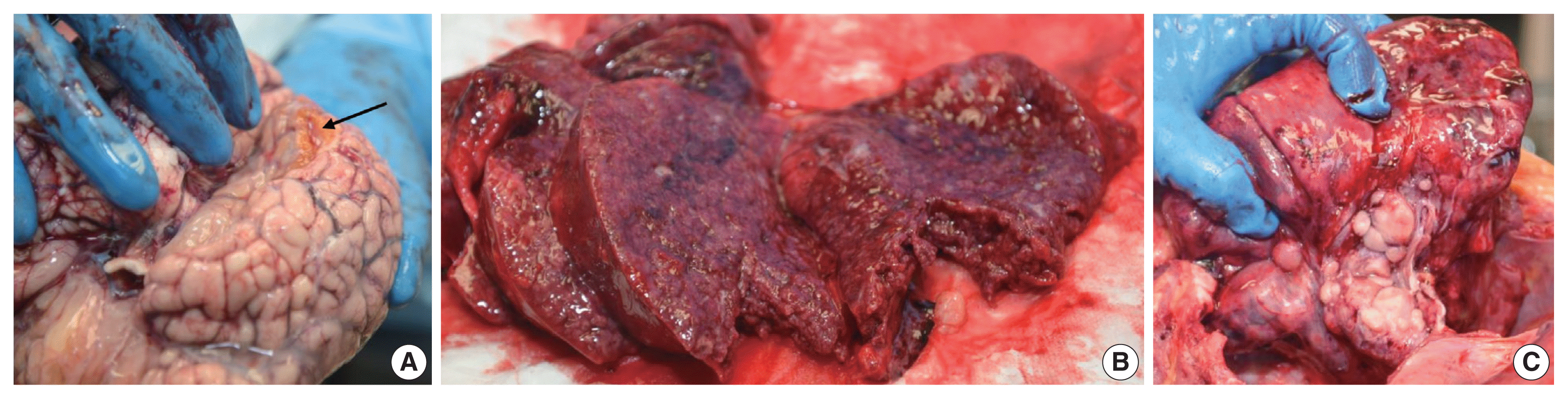
Fig. 2
Microscopic findings, pulmonary hilar lymph node (A–C) and lung (D–G). (A) Lymph node with marked paracortical expansion and vague residual follicles. (B) Neoplastic lymphocytes with a spectrum of size and morphologic atypia. (C) Positive in-situ hybridization for Epstein-Barr virus encoded RNA in neoplastic T cells. (D) Pulmonary architectural effacement by a neoplastic lymphoid infiltrate. (E) Prominent increase in T cells by immunohistochemistry (IHC) for CD3. (F) Neoplastic T cells were predominantly positive for CD4 by IHC. (G) The majority of neoplastic T cells were negative for CD8 by IHC.

Fig. 3
Additional microscopic findings. (A) Gastric mucosa diffusely infiltrated by neoplastic lymphocytes with a spectrum of size and morphologic atypia. (B) Prominent increase in T cells within the gastric mucosa by immunohistochemistry (IHC) for CD3. (C) Positive in-situ hybridization for Epstein-Barr Virus encoded RNA within the neoplastic T cells in the gastric mucosa. (D) IHC for CD163 showing hemophagocytosis by histiocytes (arrows) in the bone marrow.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download