INTRODUCTION
A microscopic ultrasound probe is widely applied in clinical practice, as it can be inserted directly into the bile and pancreatic ducts to scan lesions and surrounding structures. Intraductal ultrasonography (IDUS) using a narrow-diameter ultrasound probe provides cross-sectional images of the bile and pancreatic ducts, which can be very useful in diagnosing pancreaticobiliary disorders. The probe can be inserted into the biliary tract through the ampulla after direct contact with the lesion. An important indication for IDUS in the biliary system is the identification of the cause of biliary stenosis and determination of the extent of extrahepatic cholangiocarcinoma. It is also useful in various pancreatic diseases.
INSTRUMENTS AND INSPECTION METHODS
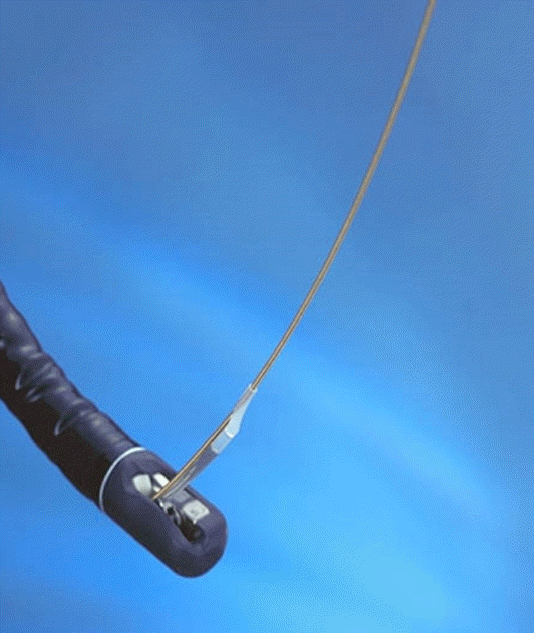
The transducer (UM-G20-29R; Olympus Co., Ltd., Tokyo, Japan) can be inserted along the guidewire (Fig. 1). The diameter, frequency, axial resolution, and maximum transmission distance of the transducer is 2.4 mm, 20 MHz, 0.1 mm, and 20 mm, respectively. Ultrasound images can be obtained using a UM-20 or UM-30 ultrasonography (US) processor. Briefly, the bile duct is selectively contrasted using an endoscopic retrograde cholangiopancreatography (ERCP) conduit. Then, the lesion (such as stenosis of the bile duct) is checked and a 0.035-inch guidewire is inserted into the bile duct above the lesion. The catheter is then removed, leaving the guidewire in the bile duct. A probe is inserted along the guidewire to the top of the lesion, and then the probe is slowly pulled out to observe the lesion and its surrounding structures. Endoscopic sphincterotomy does not need to be performed in advance to perform IDUS; however, endoscopic sphincterotomy can be performed when ERCP is performed for therapeutic purposes.
IDUS IN THE BILIARY AREA
Biliary stenosis
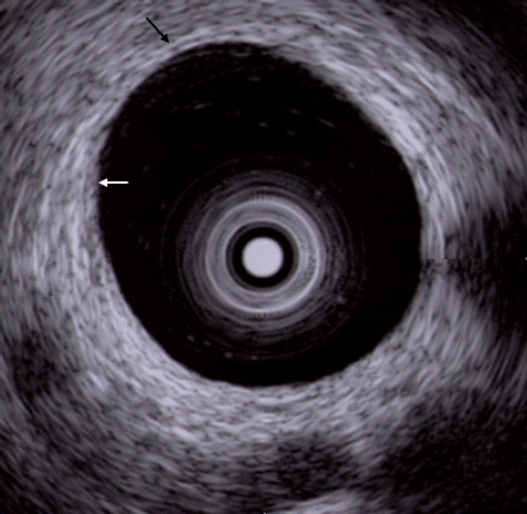
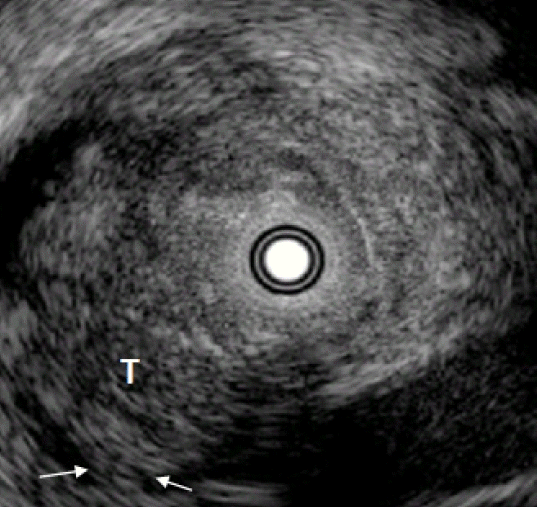
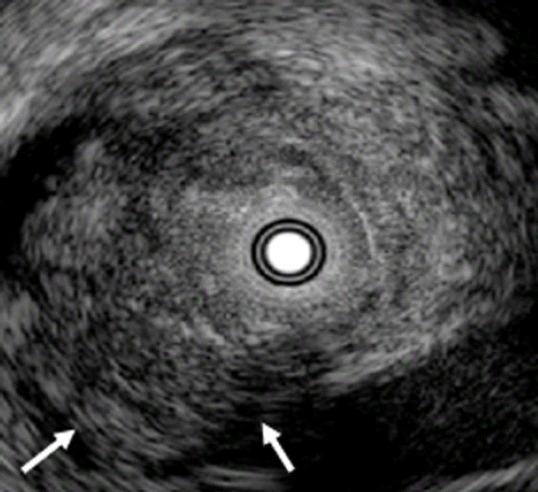
When diagnosing biliary stenosis, non-invasive methods such as ultrasound, spiral computed tomography, magnetic resonance imaging, endoscopic ultrasonography (EUS), or cholangiography are sometimes difficult to use. Thus, treatment decisions are usually based on biopsy results. However, brush cytology or biopsy, which is widely used for biliary stenosis, has limitations owing to its low sensitivity and negative predictive value for malignancy. The most accurate test method is bile duct tissue biopsy under direct cholangioscopy, and the sensitivity is reported to be 93% to 96%1,2; yet it is an invasive method. On the other hand, IDUS, administered under the guidance of a guidewire, has the advantage of easy administration and being less invasive, allowing for the observation of the biliary tract and surrounding organs. Although the penetration depth is limited to 2 cm when using a 20 MHz probe, it is sufficient to distinguish between intra-biliary lesions and extra-biliary duct compression. The accuracy of IDUS in classifying benign or malignant biliary stenosis is reported to be 76% to 92%.1,3-5 The IDUS image of the normal bile duct wall appears as two or three layers (Fig. 2). The inner low echoic layer is a fibromuscular structure, and the outer echo-rich layer consists of perimuscular connective tissue. IDUS findings suggestive of malignant biliary stenosis are as follows: (1) hypoechoic mass, especially in infiltrating surrounding tissue; (2) heterogeneity in internal echo pattern; (3) destruction of the normal bile duct wall; and (4) dentation or irregularity of the external bile duct wall and papillary projections of the inner bile duct wall (Fig. 3).1,5-7
IDUS is more accurate than EUS in determining the nature of bile duct stenosis (89% vs. 76%, respectively, p<0.002).5 Additionally, it is superior in judging whether cholangiocarcinoma can be operated on or not, and during the T stage. Moreover, IDUS is superior to EUS in evaluating tumors located in the hepatic region or common bile duct.5
Although IDUS cannot evaluate the nature of all cases of biliary stenosis, IDUS findings can be useful in determining the direction of treatment. Tamada et al.1 have previously reported that a raised tumor and destruction of the bile duct wall side is an indication for surgery, even if malignancy is not histologically proven. Furthermore, even if the bile duct wall structure is well-maintained, the possibility of malignancy is high if the diameter of the polypoid mass is ≥8 mm.
Staging and longitudinal extension of the bile duct cancer
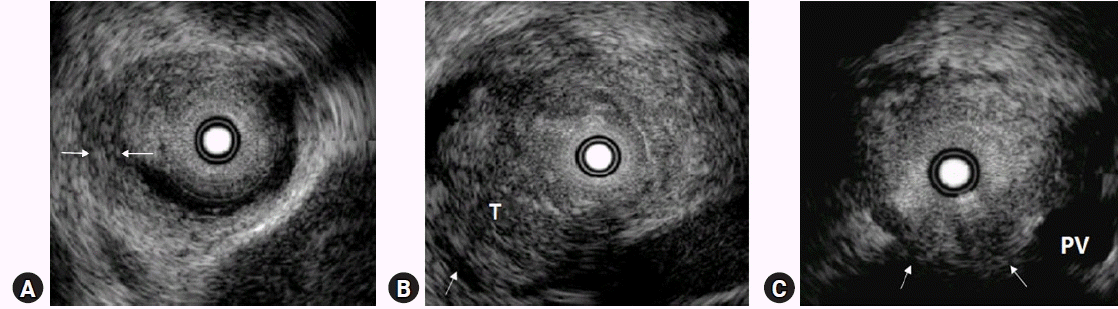
In the T1 stage of cancer, the cancer is limited to the bile duct wall and the outer margin of the tumor is smooth (Fig. 4A). In T2, the tumor invades the periductal connective tissue and interrupts the outer margin of the duct (Fig. 4B). T3 indicates tumor invasion into adjacent blood vessels (Fig. 4C). However, it is difficult to distinguish between T1 and T2 using IDUS because the fibromuscular layer and perimuscular connective tissue sometimes appear as one layer. Nevertheless, these problems have no effect on therapeutic choice. IDUS can be used to diagnose invasion of the adipose layer of the subserosa or the serosa of the bile duct wall. Menzel et al.5 reported the accuracy of IDUS in T staging to be 77.7%. Thus, the degree of expansion of the tumor in the bile duct and the determination of whether there is invasion of the surrounding blood vessels is more important in selecting treatment modalities for bile duct cancer. The longitudinal spread of bile duct cancer from the main tumor is visualized by IDUS as an irregular and asymmetric thickening of the wall. In fact, it is not easy to clearly distinguish tumors from inflammation using IDUS. Therefore, it is important to establish a standard that can differentiate between the findings caused by tumors and inflammation. The most useful finding for diagnosing inflammation is symmetric bile duct thickening, and cancer-induced thickening is often asymmetric compared to inflammation.8 Tamada et al.8 reported that asymmetric biliary wall thickening as an appropriate criterion for IDUS findings when judging intra-biliary expansion of extrahepatic cholangiocarcinoma. The accuracy of IDUS in determining the longitudinal spread of cancer toward the liver (84%) was superior to that of cholangiography (47%). However, it should be noted that in judging the degree of intra-biliary expansion of bile duct cancer, inserted stents may cause thickening of the bile duct wall if a biliary plastic or metal stent is inserted before IDUS. In fact, one study reported that 14 days after the insertion of the bile duct, the wall of the bile duct became significantly thickened because of the inflammatory reaction caused by the drainage duct.9,10 Therefore, to determine the degree of intrahepatic spread of cholangiocarcinoma with IDUS, it should be performed before insertion of the biliary drainage tube.
Biliary tract cancer frequently extends beyond the gross lesion through the submucosal spread. Extensive subepithelial tumor spread beyond the gross tumor margin is common, and longitudinal spread may extend 15 to 20 mm proximally and 5 to 10 mm distally, depending on the tumor type.11 These make it difficult to determine an appropriate surgical margin. In one case, narrowing of the common hepatic duct was observed in ERCP and magnetic resonance cholangiopancreatography findings. On IDUS, there was no evidence of tumor involvement, such as eccentric thickening in the duct of the liver hilum and invasion of the surrounding vessels. The patient underwent an extrahepatic duct excision. However, in the final pathological findings, there was tumor involvement in the liver, resulting in incomplete resection. As such, there are limitations in accurately evaluating subepithelial tumor spread with IDUS, without mucosal lesions (Fig. 5).
Vessel invasion
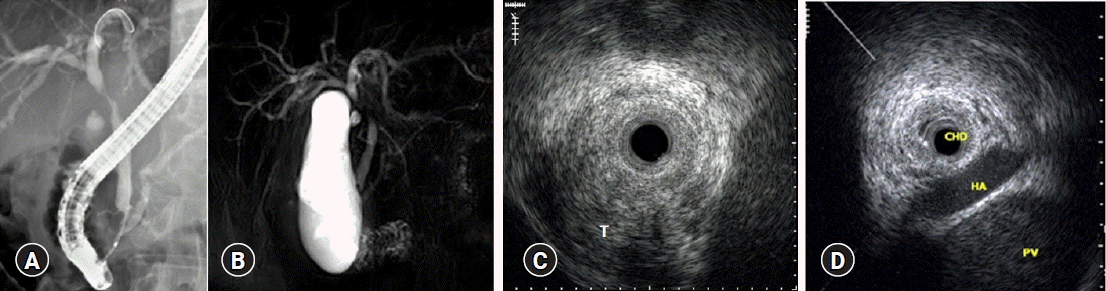
When making treatment decisions for cholangiocarcinoma, ascertaining surrounding blood vessel invasion is an important factor. The portal vein and right hepatic artery are frequently invaded, and the left hepatic artery and common hepatic artery are rarely invaded compared to the aforementioned blood vessels (Fig. 6). With IDUS, the accuracy of portal vein and right hepatic artery involvement was 100%, while angiography showed only 33% accuracy.12 However, it is difficult to determine whether the left hepatic artery and common hepatic artery are invaded because IDUS has a low penetration depth and is limited in recognition outside the hepatoduodenal ligament.13
CONCLUSIONS
IDUS is a new development in EUS technology and is of great help in the evaluation of diseases of the biliary system. It has the advantage of being technically easy to use and can be implemented immediately during ERCP. However, there is still the disadvantage that it is impossible to observe from a long distance owing to the limitation of penetration depth. IDUS provides more accurate information than other imaging methods in distinguishing between malignancy and benign biliary stenosis. Additionally, it provides information for evaluating the tumor itself and the spread of the tumor along the duct, as well as for determining the possibility of surgery. However, IDUS is not an essential diagnostic method except for the staging of bile duct tumors. Differentiation of biliary strictures should be performed by cholangioscopy or brushing cytology, as benign and malignant strictures are delineated as thickening of the bile duct wall. To conclude, IDUS is an additional technique used in ERCP studies, such as cholangioscopy and cytology.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download