1. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019; 21(Suppl 5):v1–v100. PMID:
31675094.

2. Ogasawara C, Philbrick BD, Adamson DC. Meningioma: a review of epidemiology, pathology, diagnosis, treatment, and future directions. Biomedicines. 2021; 9:319. PMID:
33801089.

3. Kim JK, Jung TY, Jung S, Lee KH, Kim SK, Lee EJ. Meningiomas with rhabdoid or papillary components: prognosis and comparison with anaplastic meningiomas. J Korean Neurosurg Soc. 2016; 59:357–362. PMID:
27446516.

4. Masalha W, Heiland DH, Delev D, Fennell JT, Franco P, Scheiwe C, et al. Survival and prognostic predictors of anaplastic meningiomas. World Neurosurg. 2019; 131:e321–e328. PMID:
31356972.

5. Palma L, Celli P, Franco C, Cervoni L, Cantore G. Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. J Neurosurg. 1997; 86:793–800. PMID:
9126894.

6. Sughrue ME, Sanai N, Shangari G, Parsa AT, Berger MS, McDermott MW. Outcome and survival following primary and repeat surgery for World Health Organization Grade III meningiomas. J Neurosurg. 2010; 113:202–209. PMID:
20225922.

7. Dziuk TW, Woo S, Butler EB, Thornby J, Grossman R, Dennis WS, et al. Malignant meningioma: an indication for initial aggressive surgery and adjuvant radiotherapy. J Neurooncol. 1998; 37:177–188. PMID:
9524097.
8. Kang H, Song SW, Ha J, Won YJ, Park CK, Yoo H, et al. A nationwide, population-based epidemiology study of primary central nervous system tumors in Korea, 2007-2016: a comparison with United States data. Cancer Res Trea. 2021; 53:355–366.

9. Hug EB, Devries A, Thornton AF, Munzenride JE, Pardo FS, Hedley-Whyte ET, et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000; 48:151–160. PMID:
11083080.
10. Kaur G, Sayegh ET, Larson A, Bloch O, Madden M, Sun MZ, et al. Adjuvant radiotherapy for atypical and malignant meningiomas: a systematic review. Neuro Oncol. 2014; 16:628–636. PMID:
24696499.

11. Orton A, Frandsen J, Jensen R, Shrieve DC, Suneja G. Anaplastic meningioma: an analysis of the National Cancer Database from 2004 to 2012. J Neurosurg. 2018; 128:1684–1689. PMID:
28731397.

12. Hassoun J. [New WHO classification of central nervous system tumors]. Bull Cancer Radiother. 1994; 81:330–334. French. PMID:
7702918.
13. Kleihues P, Sobin LH. World Health Organization classification of tumors. Cancer. 2000; 88:2887. PMID:
10870076.

14. Kobyakov GL, Absalyamova OV, Poddubskiy AA, Lodygina KS, Kobyakova EA. [The 2016 WHO classification of primary central nervous system tumors: a clinician's view]. Zh Vopr Neirokhir Im N N Burdenko. 2018; 82:88–96. Russian.

15. DeWitt JC, Mock A, Louis DN. The 2016 WHO classification of central nervous system tumors: what neurologists need to know. Curr Opin Neurol. 2017; 30:643–649. PMID:
28901970.

16. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021; 23:1231–1251. PMID:
34185076.

17. Wen PY, Packer RJ. The 2021 WHO classification of tumors of the central nervous system: clinical implications. Neuro Oncol. 2021; 23:1215–1217. PMID:
34185090.

18. Paek SH, Kim CY, Kim YY, Park IA, Kim MS, Kim DG, et al. Correlation of clinical and biological parameters with peritumoral edema in meningioma. J Neurooncol. 2002; 60:235–245. PMID:
12510775.
19. Yoshioka H, Hama S, Taniguchi E, Sugiyama K, Arita K, Kurisu K. Peritumoral brain edema associated with meningioma: influence of vascular endothelial growth factor expression and vascular blood supply. Cancer. 1999; 85:936–944. PMID:
10091773.
20. Yang SY, Park CK, Park SH, Kim DG, Chung YS, Jung HW. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry. 2008; 79:574–580. PMID:
17766430.

21. Zhao P, Hu M, Zhao M, Ren X, Jiang Z. Prognostic factors for patients with atypical or malignant meningiomas treated at a single center. Neurosurg Rev. 2015; 38:101–107. discussion 107. PMID:
25139398.

22. Choi Y, Lim DH, Jo K, Nam DH, Seol HJ, Lee JI. Efficacy of postoperative radiotherapy for high grade meningiomas. J Neurooncol. 2014; 119:405–412. PMID:
24965339.

23. Moliterno J, Cope WP, Vartanian ED, Reiner AS, Kellen R, Ogilvie SQ, et al. Survival in patients treated for anaplastic meningioma. J Neurosurg. 2015; 123:23–30. PMID:
25859807.

24. McCarthy BJ, Davis FG, Freels S, Surawicz TS, Damek DM, Grutsch J, et al. Factors associated with survival in patients with meningioma. J Neurosurg. 1998; 88:831–839. PMID:
9576250.
25. Pollock BE, Stafford SL, Link MJ, Garces YI, Foote RL. Stereotactic radiosurgery of World Health Organization grade II and III intracranial meningiomas: treatment results on the basis of a 22-year experience. Cancer. 2012; 118:1048–1054. PMID:
21773968.

26. Krayenbühl N, Pravdenkova S, Al-Mefty O. De novo versus transformed atypical and anaplastic meningiomas: comparisons of clinical course, cytogenetics, cytokinetics, and outcome. Neurosurgery. 2007; 61:495–503. discussion 503-4. PMID:
17881961.
27. Cain SA, Smoll NR, Van Heerden J, Tsui A, Drummond KJ. Atypical and malignant meningiomas: considerations for treatment and efficacy of radiotherapy. J Clin Neurosci. 2015; 22:1742–1748. PMID:
26213286.

28. Hale AT, Wang L, Strother MK, Chambless LB. Differentiating meningioma grade by imaging features on magnetic resonance imaging. J Clin Neurosci. 2018; 48:71–75. PMID:
29174756.

29. Chernov MF, Kasuya H, Nakaya K, Kato K, Ono Y, Yoshida S, et al.
1H-MRS of intracranial meningiomas: what it can add to known clinical and MRI predictors of the histopathological and biological characteristics of the tumor? Clin Neurol Neurosurg. 2011; 113:202–212. PMID:
21144647.
30. Kane AJ, Sughrue ME, Rutkowski MJ, Shangari G, Fang S, McDermott MW, et al. Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer. 2011; 117:1272–1278. PMID:
21381014.

31. Hsu CC, Pai CY, Kao HW, Hsueh CJ, Hsu WL, Lo CP. Do aggressive imaging features correlate with advanced histopathological grade in meningiomas? J Clin Neurosci. 2010; 17:584–587. PMID:
20219376.

32. Pistolesi S, Fontanini G, Camacci T, De Ieso K, Boldrini L, Lupi G, et al. Meningioma-associated brain oedema: the role of angiogenic factors and pial blood supply. J Neurooncol. 2002; 60:159–164. PMID:
12635663.
33. Coroller TP, Bi WL, Huynh E, Abedalthagafi M, Aizer AA, Greenwald NF, et al. Radiographic prediction of meningioma grade by semantic and radiomic features. PLoS One. 2017; 12:e0187908. PMID:
29145421.

34. Liu Y, Chotai S, Chen M, Jin S, Qi ST, Pan J. Preoperative radiologic classification of convexity meningioma to predict the survival and aggressive meningioma behavior. PLoS One. 2015; 10:e0118908. PMID:
25786236.
35. Buetow MP, Buetow PC, Smirniotopoulos JG. Typical, atypical, and misleading features in meningioma. Radiographics. 1991; 11:1087–1106. PMID:
1749851.

36. Nakasu S, Nakasu Y, Nakajima M, Matsuda M, Handa J. Preoperative identification of meningiomas that are highly likely to recur. J Neurosurg. 1999; 90:455–462. PMID:
10067913.

37. Helis CA, Hughes RT, Cramer CK, Tatter SB, Laxton AW, Bourland JD, et al. Stereotactic radiosurgery for atypical and anaplastic meningiomas. World Neurosurg. 2020; 144:e53–e61. PMID:
32758657.

38. Basalamah A, Al-Bolbol M, Ahmed O, Ali N, Al-Rashed S. Stereotactic radiosurgery (SRS) induced higher-grade transformation of a benign meningioma into atypical meningioma. Case Rep Surg. 2022; 2022:4478561. PMID:
35251732.

39. Shepard MJ, Xu Z, Kearns K, Li C, Chatrath A, Sheehan K, et al. Stereotactic radiosurgery for atypical (World Health Organization II) and anaplastic (World Health Organization III) meningiomas: results from a multicenter, international cohort study. Neurosurgery. 2021; 88:980–988. PMID:
33469655.
40. Hasegawa H, Vakharia K, Link MJ, Stafford SL, Brown PD, Parney IF, et al. The role of single-fraction stereotactic radiosurgery for atypical meningiomas (WHO grade II): treatment results based on a 25-year experience. J Neurooncol. 2021; 155:335–342. PMID:
34705189.

41. Kaley T, Barani I, Chamberlain M, McDermott M, Panageas K, Raizer J, et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol. 2014; 16:829–840. PMID:
24500419.

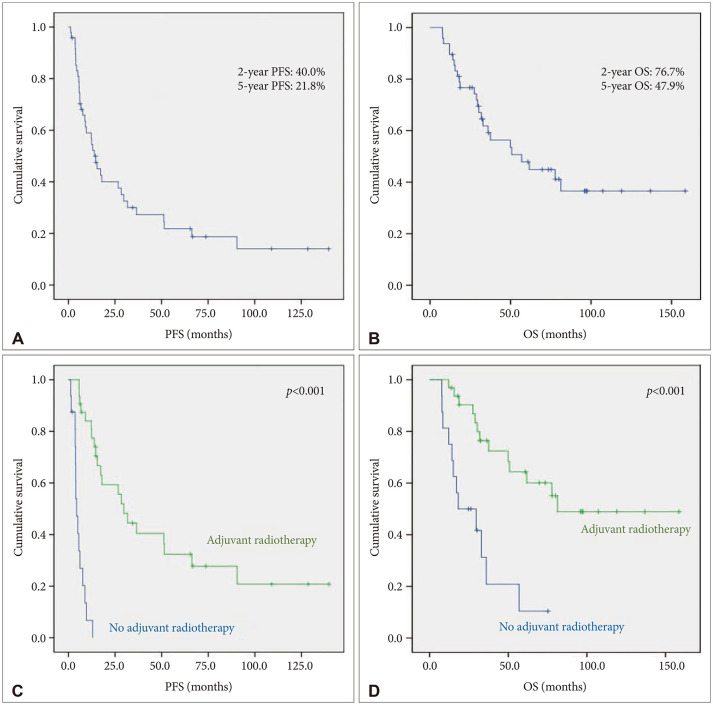
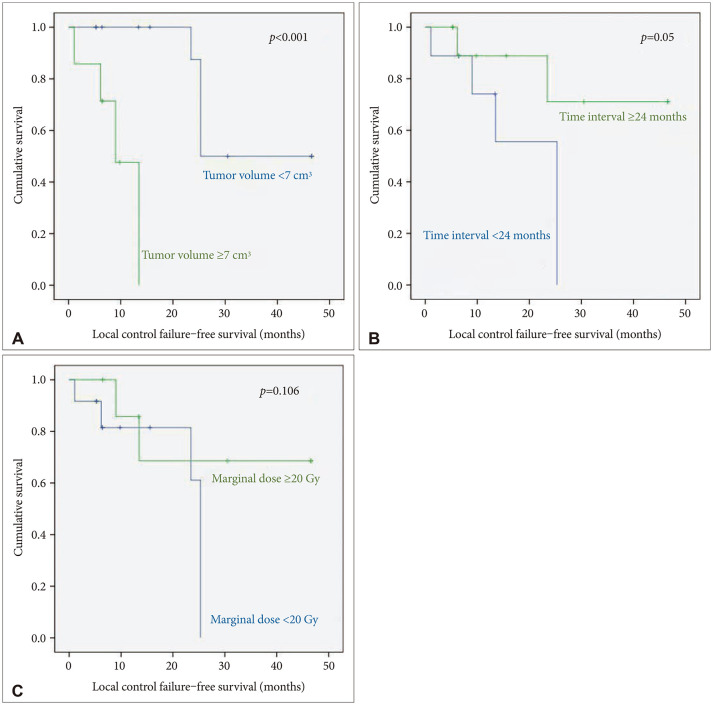
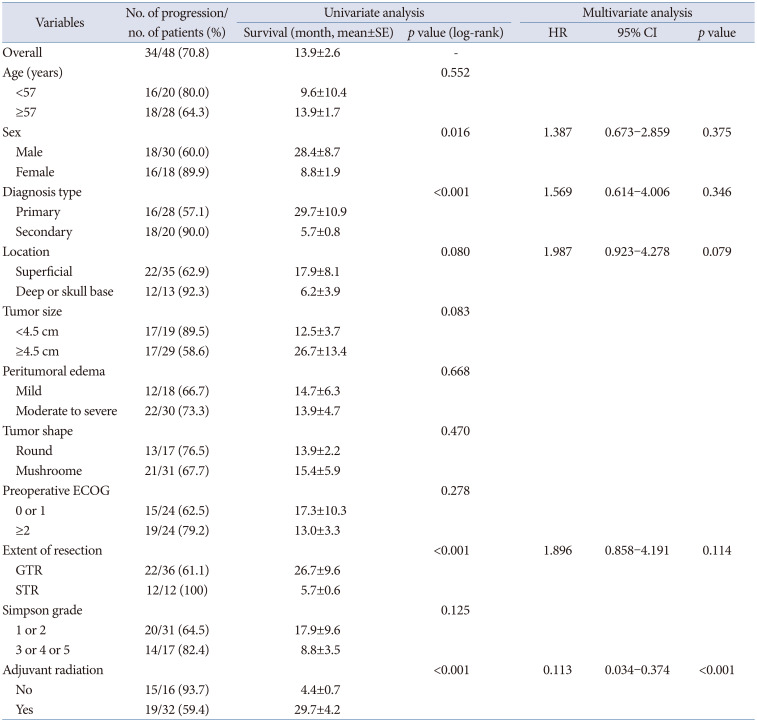




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download