Abstract
Mixed adenoneuroendocrine carcinoma is defined as a tumor with a mixture of adenocarcinoma components and neuroendocrine neoplasm components. Each of these two components of mixed adenoneuroendocrine carcinoma accounts for at least 30% of all tumors. Mixed adenoneuroendocrine carcinoma might be located in the ampulla of Vater, a very rare location compared to other organs. Thus, its treatment and prognosis plans have not been established yet. We report three cases of mixed adenoneuroendocrine carcinoma occurring in the ampulla of Vater. Each patient had a different clinical course. In general, difficulty in preoperative diagnosis, risk of early recurrence, and poor disease course were main hallmarks of mixed adenoneuroendocrine carcinoma arising from the ampulla of Vater. However, one patient in this case report survived although she did not receive adjuvant chemotherapy due to her old age. Therefore, it is important to establish a careful treatment strategy for mixed adenoneuroendocrine carcinoma arising from the ampulla of Vater.
Since the first report of gastrointestinal tumors with exocrine and neuroendocrine components by Cordier in 1924, various cases in various organs have been published. In 2010, the World Health Organization (WHO) proposed a standardized classification system for neuroendocrine tumors (NETs) based on a mitotic index representing 10 times high-power fields (HPF) per mitoses and Ki-67%. This tumor previously referred to by a variety of names was first defined as a mixed adenoneuroendocrine carcinoma (MANEC). MANEC is defined as a tumor in which adenocarcinoma components and neuroendocrine neoplasm components are mixed. Each component may theoretically exist in varying proportions. In 2010, WHO defined that these two components of MANEC must each account for at least 30% of all tumors.
MANEC can occur in various digestive organs where NETs can develop [1]. However, it rarely occurs in the ampulla of Vater (AOV). Only 21 such cases have been reported in the English literature. Due to the rarity of this tumor, there are no established cohort studies or original articles on its treatment or prognosis.
In this report, we describe three cases of MANEC occurring in the AOV. By introducing this rare condition and outlining its diagnosis, course of treatment, and prognosis, this report might contribute to the development of a treatment strategy for effective management of this condition in the future.
Publication of this study was approved by the Institutional Review Board of Asan Medical Center (ID: S2021-1895-0001).
A mass-like lesion in the AOV was discovered in a 65-year-old female incidentally by computed tomography (CT) scan performed during a medical examination. The patient was referred to our hospital for further evaluation. The underlying disease was hypertension and hyperlipidemia. The patient had no family history of cancer. However, she was a heavy drinker for more than 20 years. In addition, she had undergone wedge resection of the stomach for a gastrointestinal stromal tumor (GIST). There were no symptoms such as abdominal pain, jaundice, or dark urine. There were no specific findings when system and physical examinations at the time of admission were reviewed.
Magnetic resonance cholangiopancreatography (MRCP) was performed (Fig. 1). Positron emission tomography–computed tomography (PET-CT) revealed an elevated uptake (standardized maximum uptake value 8.0) in the AOV. However, there were no findings suggestive of distant metastasis.
Biopsy was performed on the ampullary lesion, which was subsequently diagnosed as a MANEC, a mixture of a small cell-type neuroendocrine tumor and poorly differentiated adenocarcinoma. The lesion was judged to be resectable. Laparoscopic pylorus-preserving pancreaticoduodenectomy (PPPD) was performed on September 28, 2020 (Fig. 1). There were no unusual findings during surgery. We discovered a well-defined, irregular, solid mass (2 cm × 1.7 cm × 1.5 cm) in the AOV, which had extended to the pancreas parenchyma, common bile duct, and proper muscle of the duodenum. Pathologic findings of the resected specimen showed that the tumor had two elements: small cell components in the neuroendocrine carcinoma (mitotic rate of 8/10 HPFs, Ki-67 labeling index of 86%, and CD56 staining positive) and a poorly differentiated adenocarcinoma (Fig. 2). The tumor was assessed as T3bN2 (7/20), stage IIIB according to the AJCC 8th standard stage. Lymphovascular invasion was present. However, perineural invasion was not observed. Surgical resection margins were free of tumor. The patient’s condition improved after endoscopic ultrasonography-guided gastrocystostomy with postoperative pancreatic fistula grade B. The patient has been receiving chemotherapy (Leucovorin plus 5-fluorouracil) since November 23, 2020 without recurrence to date.
An 81-year-old male presented with complaints of jaundice, poor oral intake, and general weakness. A mass-like lesion in the AOV was discovered on an outside-hospital CT image. The patient was referred to our hospital for further evaluation. The patient had no specific underlying disease. He had no family history of cancer. MRCP were performed (Fig. 3). No findings were suggestive of distant metastasis in PET-CT.
An endoscopic biopsy was performed on the ampullary lesion. However, no cancer was diagnosed. Because AOV cancer was strongly suspected clinically, PPPD was performed on June 27, 2014. We discovered an ill-defined, irregular, solid mass (3.5 cm × 1.5 cm × 1.0 cm) with hemorrhagic change in the AOV, which had extended to the common bile duct. Pathologic findings of the resected specimen showed that the tumor had two elements: large cell components in the neuroendocrine carcinoma (mitotic rate of 36/10 HPFs and Ki-67 labeling index of 80%), with remaining components showing a moderately differentiated adenocarcinoma. The AJCC 8th standard stage was T3aN1 (2/30), stage IIIA. Lymphovascular and perineural invasions were not observed. The surgical resection margin was free of tumor. No chemotherapy was performed due to the patient’s advanced age. The prognosis of patient was not expected to be good. However, the patient is still alive. He has been followed up without recurrence to date.
A 73-year-old female presented with complaints of dark urine, pruritus, and epigastric discomfort. As jaundice worsened, a CT scan was performed. A mass-like lesion in the AOV was discovered and the patient was referred to our hospital. The underlying disease was hypertension. The patient had no family history of cancer. MRCP was performed (Fig. 4). PET-CT revealed an elevated uptake at the AOV. PET-CT showed no findings suggestive of distant metastasis.
Endoscopic biopsy was performed. Pathology revealed atypical cells. On June 8, 2017, PPPD was performed due to a strong clinical suspicion of AOV cancer. We discovered an ill-defined, firm, solid mass (1.5 cm × 1.4 cm × 0.6 cm) in the AOV, which had extended to the common bile duct and proper muscle of duodenum. Pathologic findings of the resected specimen showed that the tumor had two elements: neuroendocrine carcinoma (Ki-67 labeling index of 90%, chromogranin positive, and synaptophysin positive) and a moderately differentiated adenocarcinoma. According to the AJCC 8th standard stage, the tumor was defined as T2N1 (1/21), stage IIIA. Lymphovascular and perineural invasions were present. The surgical resection margin was free of tumor. The patient received chemotherapy (Leucovorin plus 5-fluorouracil) from July 12, 2017 to November 3, 2017. However, recurrence was confirmed in the liver and para-aortic lymph nodes on November 18, 2017. The patient succumbed to disease progression on December 27, 2018. Table 1 shows laboratory findings of the three patients. Pathological and clinical outcomes of the three cases are summarized in Table 2.
Table 3 [2-16] shows previous studies of MANEC of AOV. The age of patients ranged from 27 to 89 years. The male to female ratio was 8 : 10. Preoperative MANEC was diagnosed in only two cases. The remaining 14 cases were diagnosed as AOV cancer. Pancreaticoduodenectomy was performed in 17 cases. Lymph node metastasis was confirmed in eight cases. The mean survival time was 15 months. Six deaths were confirmed. In another study, the median survival of AOV MANEC patients was 13.2 months [17]. However, the median survival of AOV adenocarcinoma was reported to be 49.3 months [17]. It was found to be 60.2 months in our hospital data [17].
MANEC occurring in the AOV does not cause any specific symptoms until the disease has progressed. Carcinoid symptoms are rare. The most common symptom of the disease is jaundice caused by blockage of the bile duct due to progression of the mass. Other symptoms include non-specific abdominal pain, weight loss, and anorexia. If MANEC in the AOV is suspected, imaging tests involving the pancreas and biliary tract are performed. CT and MRCP can identify a mass-like lesion that invades the AOV. This method can confirm whether the bile duct or pancreatic duct is enlarged. However, since there are no typical radiographic findings of MANEC, it is rarely diagnosed before surgery, with most cases originally diagnosed as adenocarcinoma of AOV. Additionally, there is a limitation in that only one of these two components of MANEC can be obtained through a preoperative biopsy. Therefore, it might not be possible to distinguish between each pure component of MANEC before surgery. The majority of diagnoses made after a biopsy are adenocarcinomas because the neuroendocrine tumor component is deeply located while the adenocarcinoma component typically has superficial occurrence [2]. In two of the three cases presented in our case report, biopsy results indicated preoperative adenocarcinoma. MANEC was diagnosed in only one recent case. Although it is difficult to make an accurate diagnosis of preoperative MANEC, a combination of endoscopic ultrasound-guided fine needle aspiration with immunohistochemistry and deep biopsy can improve this accuracy. Therefore, a deep biopsy should be considered if there is a slight heterogeneous contrast effect in imaging findings. In addition, when diagnosing MANEC in postoperative specimens, tumor immunohistochemical analysis using three neuroendocrine markers (CD56, chromogranin, and synaptophysin) should be performed. For the patient in case 1, the resected tumor sample was CD56 positive and the other two were negative. However, in case 3, all three markers were positive. In the diagnosis of MANEC, two out of three neuroendocrine markers must be expressed in abundance to make a definitive diagnosis of MANEC.
According to morphological components, MANEC can be classified into three types [18]. The amphicrine type refers to a single cell type with both endocrine and exocrine characteristics by proliferating in early stem cells before differentiation into endocrine and exocrine lineages. When early stem cells differentiate into primordial endocrine cells and exocrine cells, two types can arise. A composite type is a form in which cells of two lineages are mixed, with one dominant and one minor component coexisting. The collision type refers to a type composed of an endocrine component at one end and an exocrine component at the other, with connective tissue in the middle separating them. According to our morphological and immunohistochemical examinations, case 1 presented in our report could be considered as having a collision tumor.
Considering the heterogeneity of this type of tumor, it has been proposed that clinicians should group these tumors as follows using results to indicate different prognoses according to the malignancy class of each component. High grades are combined with G3 component and adenoma or carcinoma components. They are generally more aggressive. Intermediate grades consist of carcinoma and G1 and G2 combined or amphicrine carcinoma. The prognosis is usually determined by the non-neuroendocrine component, not the NET component. Low grades are a combination of adenoma and G1-G2. With the exception of low grade cases, MANEC is generally considered to have a poor prognosis [18]. In our case, there was no significant difference between patients in terms of clinical symptoms or time of diagnosis. However, all patients had lymph node metastasis. In addition, the survival rate was worse than that of AOV cancer (adenocarcinoma).
MANEC occurs mainly in the colon/rectum, stomach, and pancreas. Occurrence in the biliary tract, including the AOV, is relatively rare. This is because neuroendocrine cells are rarely identified in the normal biliary tract. The gallbladder is the location of occurrence more frequently than the extrahepatic bile duct because intestinal metaplasia from chronic cholecystitis and cholelithiasis is more common in the gallbladder. MANEC in the gallbladder is reported to be approximately twice as common in females. It is the most common in Asian patients. Median overall survival was observed for about 12.2 months. Four recurrences and two deaths were confirmed during the observation period [19]. MANEC identified in the duodenum is often diagnosed by combining cell components with relatively high differentiation. Therefore, they are non-superficial and non-aggressive with fewer distant metastasis sites than MANEC that occurs in other periampullary or biliary tracts. The pancreas is a more well-known site of MANEC than the biliary tract or duodenum. In general, large cell types of the NET component and ductal adenocarcinoma, or rarely acinar cell carcinoma, or a combination of all three, are common. It is known that the prognosis is slightly worse than that of MANEC, which occurs in the duodenum [1].
Since MANEC has few cases, an exact treatment has not been established yet [3]. However, the main framework of treatment is mostly identified as neuroendocrine carcinoma (NEC) components when diagnosed with MANEC [1]. NEC components are regarded as being more commonly involved in lymphovascular invasion, perineural invasion, and lymph node metastasis than adenocarcinoma. They are considered to have high invasiveness and poor prognosis. In the case of AOV MANEC, PPPD is performed to achieve curative resection, while chemotherapy targets the NEC component. The most important aspect in the treatment of non-metastatic MANEC is whether surgical resection is possible before or after chemotherapy. If adjuvant treatment is required or surgical resection is not possible, it is divided according to the malignancy grade of each component. Treatment plan is determined according to the adenocarcinoma component in the case of intermediate-grade MANEC. In the case of high-grade, treatment plan is determined according to the NEC component. Generally, a regimen that includes platinum-based chemotherapy is used to treat the MANEC of AOV (e.g., irinotecan + cisplatin or etoposide + cisplatin). In our case, LV5FU2 was administered except for patients who had not undergone chemotherapy due to their advanced age. The patient's disease-free survival was six months with overall survival of 16 months. Case 1 patient is still alive. She has been followed up without recurrence to date. One study has reported that the patient treated for six months with TS-1 as adjuvant chemotherapy is alive without recurrence after surgery [20]. Since there is no definitively established chemotherapy regimen, it is important to confirm reports of various cases and proceed further.
In the treatment of metastatic MANEC, components present within the metastasized area should be targeted according to each malignancy grade. Treatment according to each malignancy grade is the same as for non-metastatic MANEC. The prognosis of MANEC arising from AOV remains controversial because of the rarity of the tumor. However, the NET component of MANEC arising from the biliary tract defines the prognosis. In addition, the NEC component and its Ki-67 labeling index are reportedly the main prognostic factors in MANEC. There is a report that if the Ki-67 index level is 55% or higher, the mortality rate increases more than 8-fold [3,16]. In addition, high-grade NEC of AOV reportedly shows a more aggressive clinical course than locally advanced adenocarcinoma of AOV. However, as shown in case 2, there might be cases where patients with Grade 3 NEC and Ki-67 values greater than 80% can survive for more than five years without chemotherapy.
We report three cases of AOV MANEC with various characteristics and prognoses. Generally, difficulty in preoperative diagnosis, risk of early recurrence, and poor disease course are main characteristics of this cancer. Therefore, establishing a careful therapeutic strategy is crucial. In the future, it will be necessary to establish an appropriate treatment strategy based on reports of many cases.
REFERENCES
1. De Mestier L, Cros J, Neuzillet C, Hentic O, Egal A, Muller N, et al. 2017; Digestive system mixed neuroendocrine-non-neuroendocrine neoplasms. Neuroendocrinology. 105:412–425. DOI: 10.1159/000475527. PMID: 28803232.

2. Vilardell F, Velasco A, Cuevas D, Olsina JJ, Matias-Guiu X. 2011; Composite papillary intestinal-type adenocarcinoma/poorly differentiated neuroendocrine carcinoma of the ampulla of Vater. J Clin Pathol. 64:174–177. DOI: 10.1136/jcp.2010.084954. PMID: 21097790.

3. Yoshimachi S, Ohtsuka H, Aoki T, Miura T, Ariake K, Masuda K, et al. 2020; Mixed adenoneuroendocrine carcinoma of the ampulla of Vater: a case report and literature review. Clin J Gastroenterol. 13:37–45. DOI: 10.1007/s12328-019-01009-2. PMID: 31342462.

4. Jones MA, Griffith LM, West AB. 1989; Adenocarcinoid tumor of the periampullary region: a novel duodenal neoplasm presenting as biliary tract obstruction. Hum Pathol. 20:198–200. DOI: 10.1016/0046-8177(89)90187-1. PMID: 2914703.

5. Misonou J, Kanda M, Kitagawa T, Ota T, Muto E, Nenohi M, et al. 1990; A case of coexisting malignant carcinoid tumor and adenocarcinoma in the papilla of Vater. Gastroenterol Jpn. 25:630–635. DOI: 10.1007/BF02779365. PMID: 2227254.

6. Burke A, Lee YK. 1990; Adenocarcinoid (goblet cell carcinoid) of the duodenum presenting as gastric outlet obstruction. Hum Pathol. 21:238–239. DOI: 10.1016/0046-8177(90)90137-T. PMID: 2307453.

7. Shah IA, Schlageter MO, Boehm N. 1990; Composite carcinoid-adenocarcinoma of ampulla of Vater. Hum Pathol. 21:1188–1190. DOI: 10.1016/0046-8177(90)90158-2. PMID: 2227927.

8. Williams IM, Williams NW, Stock D, Foster ME. 1997; Collision tumour of the ampulla of Vater: carcinoid and adenocarcinoma. HPB Surg. 10:241–244. DOI: 10.1155/1997/46751. PMID: 9184878. PMCID: PMC2423872.

9. Alex WR, Auerbach HE, Pezzi CM. 1998; Adenocarcinoid tumor of the ampulla of Vater. Am Surg. 64:355–359.
10. Moncur JT, Lacy BE, Longnecker DS. 2002; Mixed acinar-endocrine carcinoma arising in the ampulla of Vater. Hum Pathol. 33:449–451. DOI: 10.1053/hupa.2002.124040. PMID: 12055683.

11. Nassar H, Albores-Saavedra J, Klimstra DS. 2005; High-grade neuroendocrine carcinoma of the ampulla of vater: a clinicopathologic and immunohistochemical analysis of 14 cases. Am J Surg Pathol. 29:588–594. DOI: 10.1097/01.pas.0000157974.05397.4f. PMID: 15832081.
12. Deschamps L, Dokmak S, Guedj N, Ruszniewski P, Sauvanet A, Couvelard A. 2010; Mixed endocrine somatostatinoma of the ampulla of Vater associated with a neurofibromatosis type 1: a case report and review of the literature. JOP. 11:64–68.
13. Zhang L, DeMay RM. 2014; Cytological features of mixed adenoneuroendocrine carcinoma of the ampulla: two case reports with review of literature. Diagn Cytopathol. 42:1075–1084. DOI: 10.1002/dc.23107. PMID: 24554593.

14. Huang Z, Xiao WD, Li Y, Huang S, Cai J, Ao J. 2015; Mixed adenoneuroendocrine carcinoma of the ampulla: two case reports. World J Gastroenterol. 21:2254–2259. DOI: 10.3748/wjg.v21.i7.2254. PMID: 25717267. PMCID: PMC4326169.

15. Ginori A, Lo Bello G, Vassallo L, Tripodi SA. 2015; Amphicrine carcinoma of the ampullary region. Tumori. 101:e70–e72. DOI: 10.5301/tj.5000254. PMID: 25702653.

16. Li X, Li D, Sun X, Lv G. 2020; Mixed adenoneuroendocrine carcinoma (MANEC) of the ampulla of Vater in a Chinese patient: a case report. J Int Med Res. 48:300060520947918. DOI: 10.1177/0300060520947918. PMID: 32833541. PMCID: PMC7448144.

17. Doepker MP, Thompson ZJ, Centeno BA, Kim RD, Wong J, Hodul PJ. 2016; Clinicopathologic and survival analysis of resected ampullary adenocarcinoma. J Surg Oncol. 114:170–175. DOI: 10.1002/jso.24281. PMID: 27158031. PMCID: PMC7771532.

18. La Rosa S, Marando A, Sessa F, Capella C. 2012; Mixed adenoneuroendocrine carcinomas (MANECs) of the gastrointestinal tract: an update. Cancers (Basel). 4:11–30. DOI: 10.3390/cancers4010011. PMID: 24213223. PMCID: PMC3712682.

19. Acosta AM, Wiley EL. 2016; Primary biliary mixed adenoneuroendocrine carcinoma (MANEC): a short review. Arch Pathol Lab Med. 140:1157–1162. DOI: 10.5858/arpa.2015-0102-RS. PMID: 27684986.

20. Hatano H, Yoneyama C, Noda T, Tomimaru Y, Hirota M, Takata A, et al. 2015; [A case of surgical treatment for mixed adenoneuroendocrine carcinoma of the ampulla of Vater]. Gan To Kagaku Ryoho. 42:1755–1757. Japanese.
Fig. 1
(A) Case 1 magnetic resonance cholangiopancreatography (MRCP) findings of the neoplastic lesion showing bile duct dilatation, obstruction in the ampullary area of the stretched bile duct, a 2.3 cm long mass involving the ampulla of Vater, and lymph nodes less than 1 cm in the retropancreatic area. (B) Case 1 surgical specimen of the resected ampullary tumor.
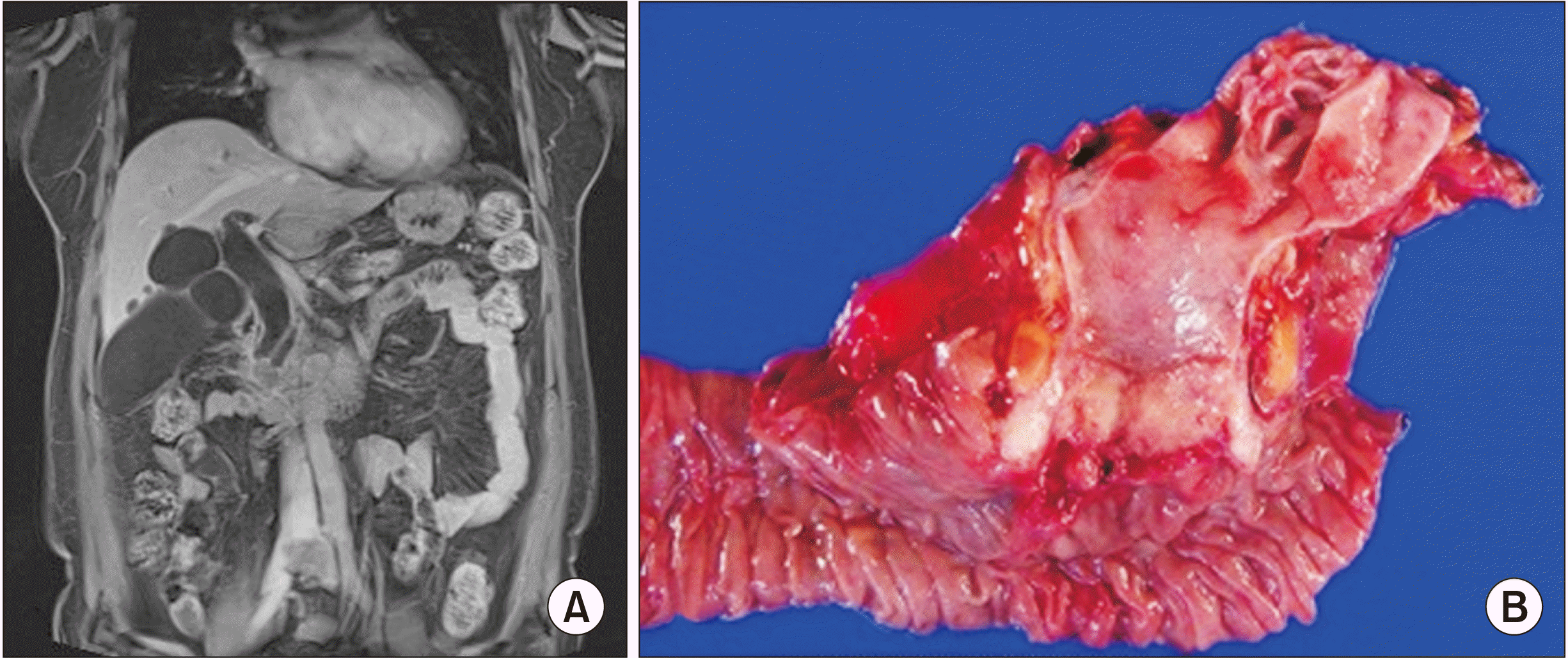
Fig. 2
(A) Case 1 H&E staining showing a mixed adenoneuroendocrine tumor (x200). (B) Case 1 immunohistochemical analysis showing neuroendocrine tumor cells being positive for CD56 (x200).
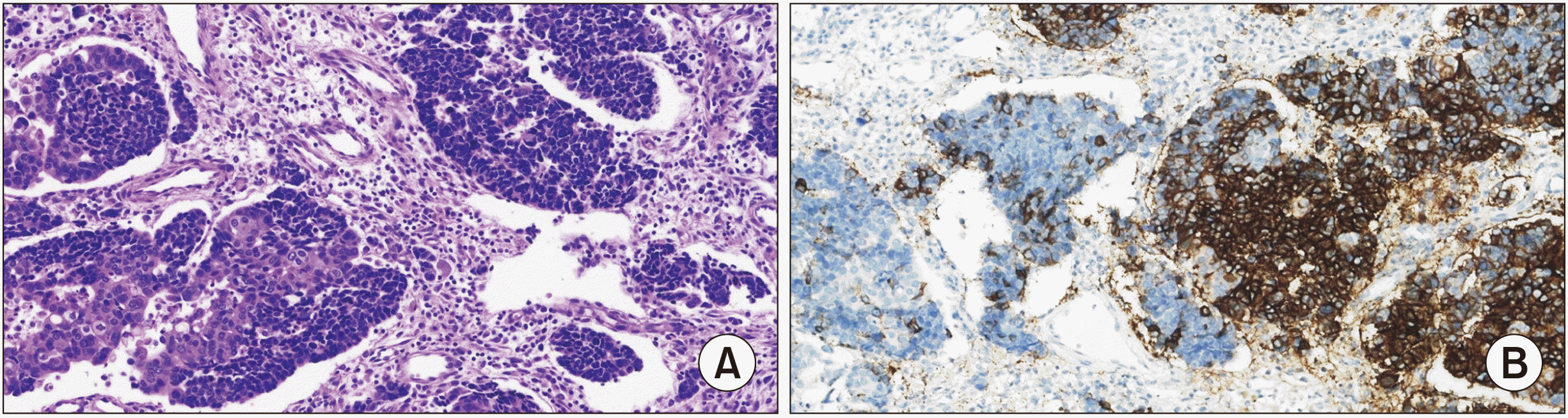
Fig. 3
(A) Case 2 magnetic resonance cholangiopancreatography (MRCP) findings of the neoplastic lesion showing bile duct dilatation, obstruction in the ampullary area of the stretched bile duct, and a 3.0 cm long mass involving the ampulla of Vater without enlarged lymph nodes. (B) Case 2 surgical specimen of the resected ampullary tumor.
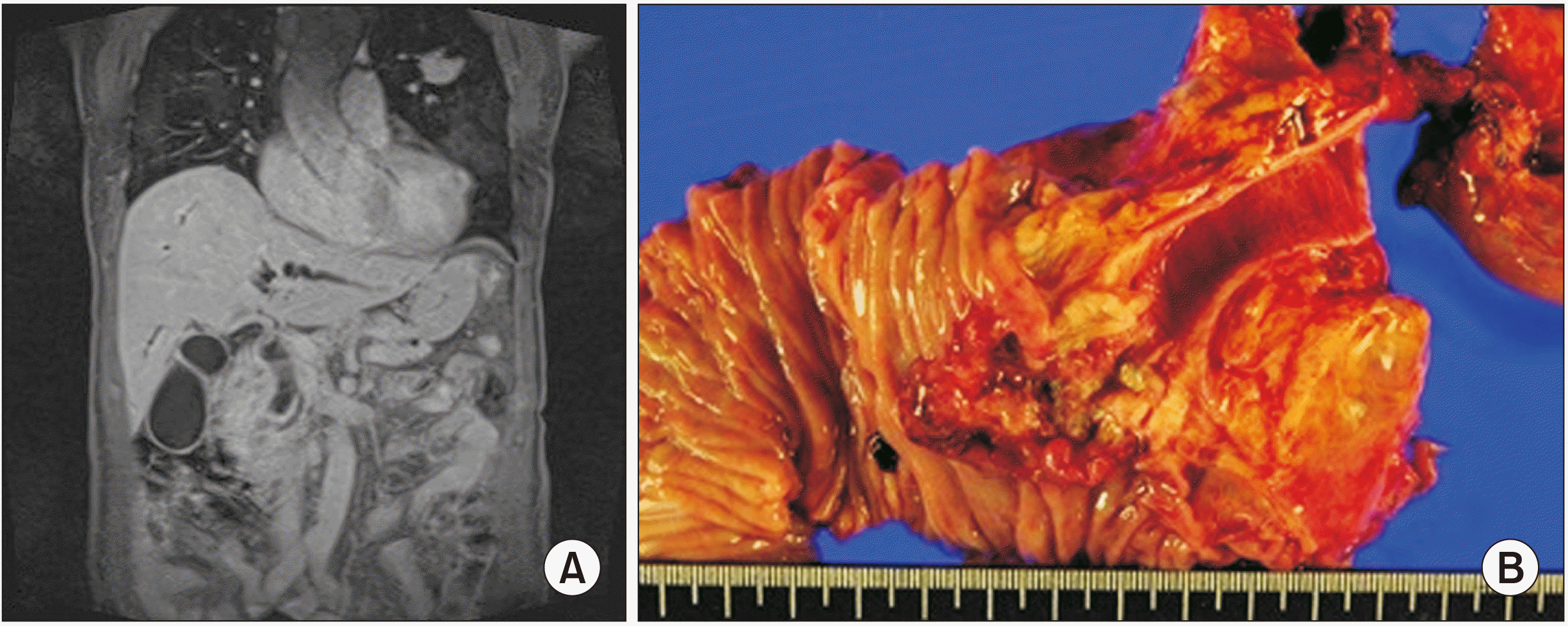
Fig. 4
(A) Case 3 magnetic resonance cholangiopancreatography (MRCP) findings of the neoplastic lesion showing bile duct dilatation, obstruction in the ampullary area of the stretched bile duct and main pancreatic duct dilatation, and a 1.5 cm long mass involving the ampulla of Vater without enlarged lymph nodes. (B) Case 3 surgical specimen of the resected ampullary tumor.
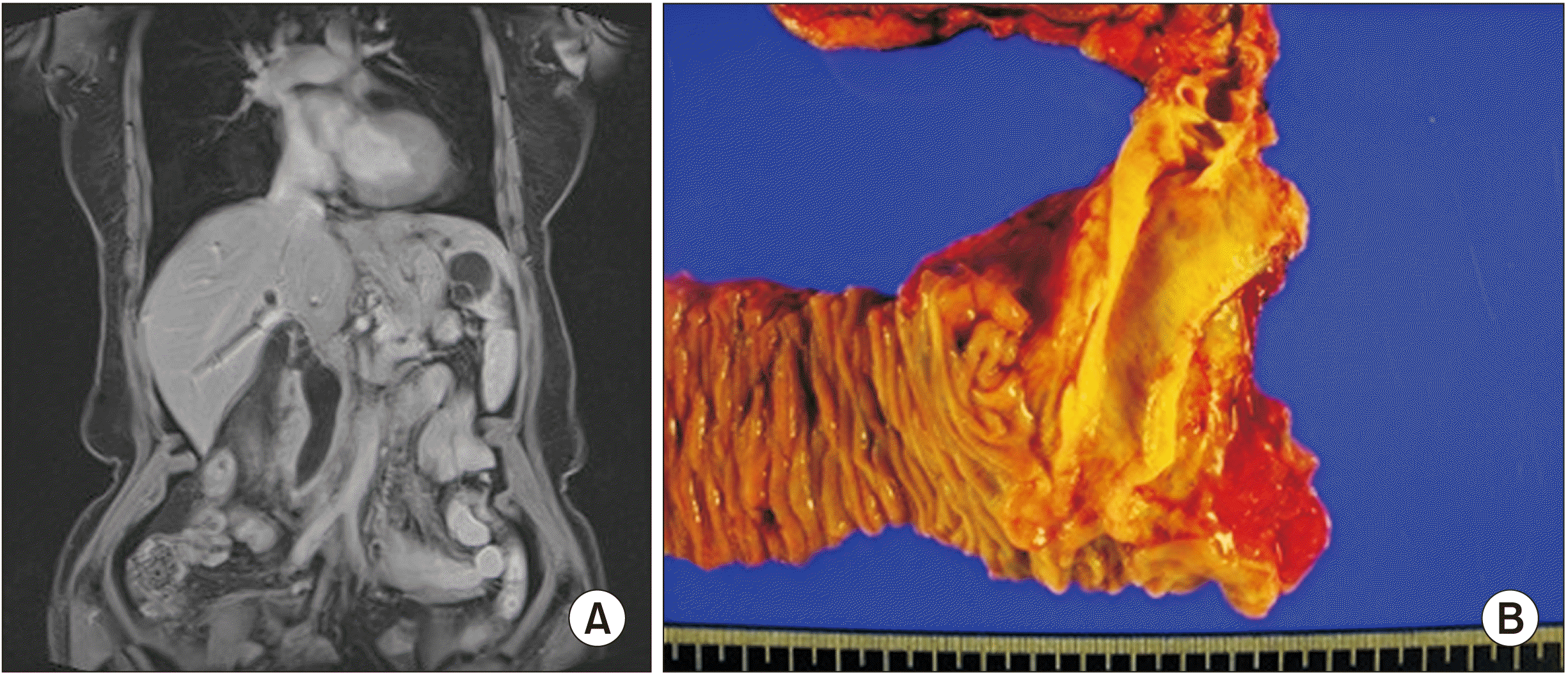
Table 1
Laboratory characteristics of patients in this case report
Table 2
Pathologic and clinical characteristics of patients in this case report
Table 3
Summary of mixed adenoneuroendocrine carcinoma of the ampulla of Vater in the literature
| Authors | Age (yr) | Sex | Preoperative diagnosis | Adenocarcinoma differentiation | NET grade | Lymph node | Death | Outcome (mon) |
|---|---|---|---|---|---|---|---|---|
| Jones et al. [4] | 64 | F | ADC | Well | G1 | Negative | Alive | 35 |
| Misonou et al. [5] | 47 | F | ADC | Well | G3 | Positive | Alive | 9 |
| Burke and Lee [6] | 45 | M | ADC | Well | G1 | Negative | Alive | 24 |
| Shaw et al. [7] | 27 | F | ADC | Moderately-poorly | G1 | Negative | Death | |
| Williams et al. [8] | 58 | F | MANEC | Moderate | G1-2 | Positive | Alive | Not noted |
| Alex et al. [9] | 63 | F | ADC | Well-moderately | G1-2 | Negative | Alive | 24 |
| Moncur et al. [10] | 78 | M | ADC | Acinar cell | G3 | Negative | Death | 2 |
| Nassar et al. [11] | 89 | M | ADC | Moderately | G3 | Negative | Alive | 6 |
| 69 | F | ADC | Moderately | G3 | Negative | Not noted | ||
| Deschamps et al. [12] | 49 | F | ADC | Well | G1 | Positive | Alive | 36 |
| Vilardell et al. [2] | 81 | M | ADC | Moderately | G3 | Positive | Not noted | |
| Zhang et al. [13] | 69 | M | ADC | Well-moderately | G3 | Negative | Alive | 33 |
| 60 | M | MANEC | Poorly | G3 | Positive | Alive | 9 | |
| Huang et al. [14] | 43 | F | ADC | Poorly | G3 | Negative | Death | 20 |
| 60 | F | ADC | Poorly | G3 | Negative | Death | 15 | |
| Ginori et al. [15] | 69 | M | Not noted | Not noted | G2 | Positive | Alive | 12 |
| Xu Li et al. [16] | 57 | M | Not noted | Moderately | G3 | Positive | Death | 14 |
| Yoshimachi et al. [3] | 75 | F | ADC | Moderate-poorly | G3 | Positive | Death | 10 |
| Case 1 | 65 | F | MANEC | Poorly | G3 | Positive | Alive | 9 |
| Case 2 | 81 | M | Not noted | Moderately | G3 | Positive | Alive | 84 |
| Case 3 | 73 | F | Not noted | Moderately | NA | Positive | Death | 16 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download