Abstract
Objective
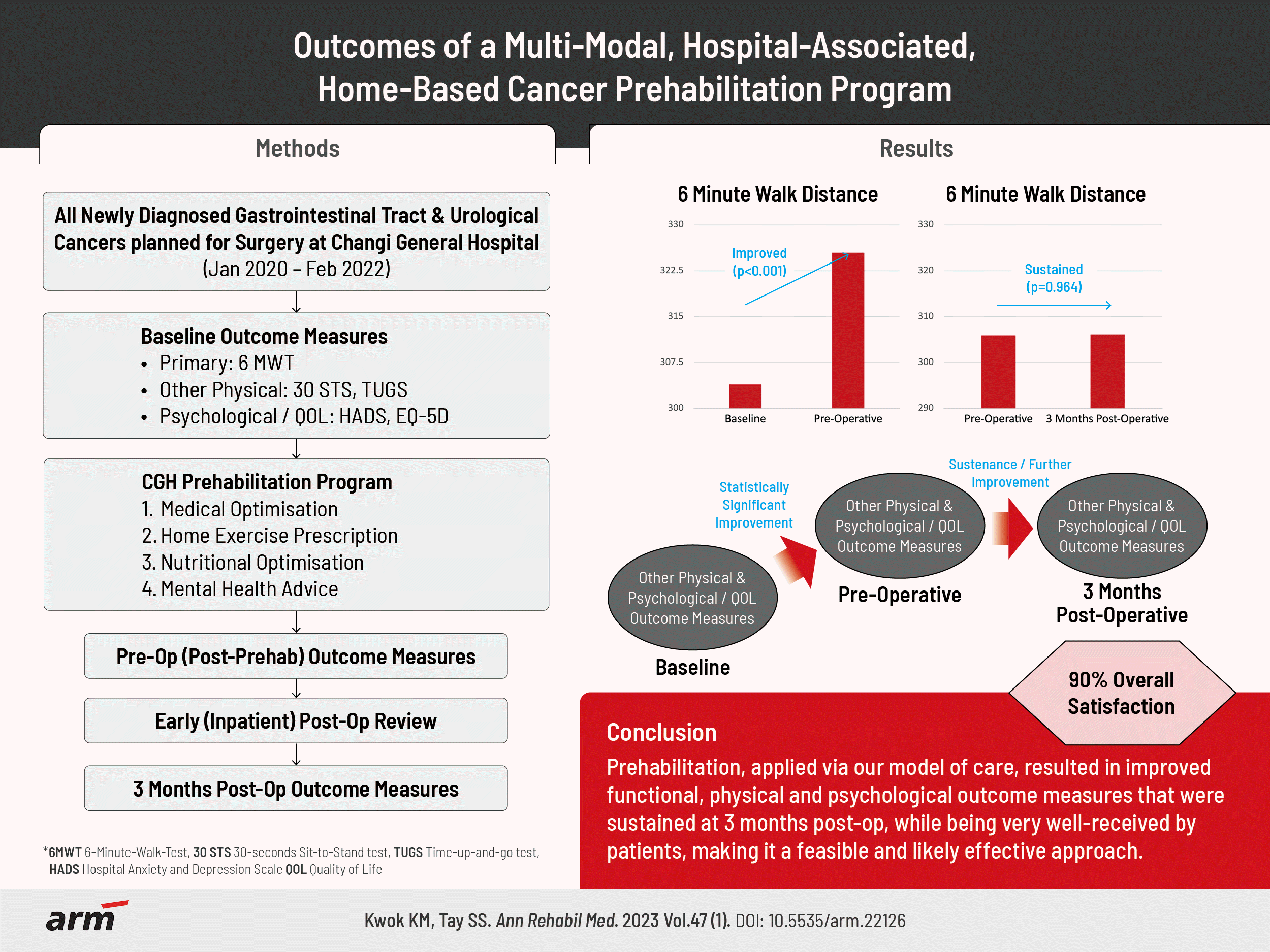
Methods
Results
ACKNOWLEDGMENTS
REFERENCES
Fig. 1.
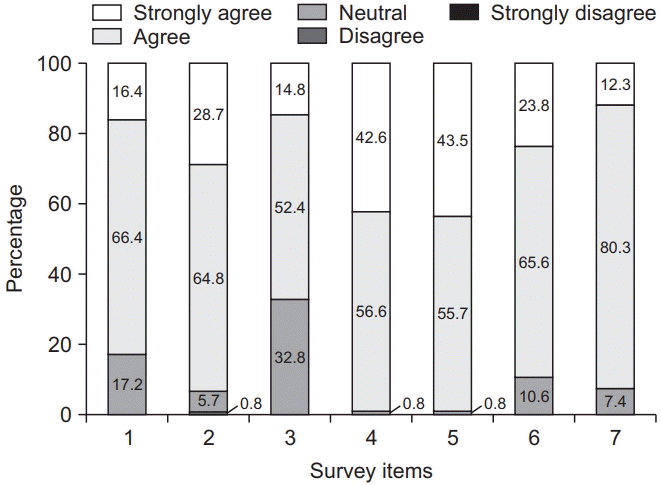
Fig. 2.
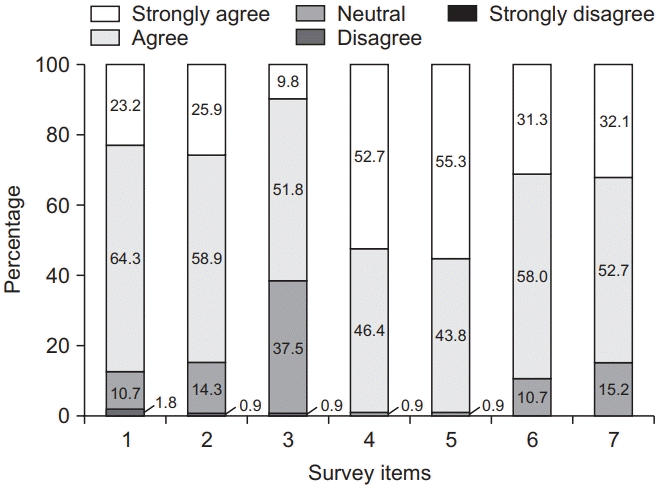
Table 1.
| Outcome measure | Collection time points | |
|---|---|---|
| Functional (physical) | Baseline | |
| 6-minute walk test (primary) | Pre-op (post-prehab) | |
| 30-Second Sit to Stand test | 3-month follow-up | |
| Timed Up and Go test | ||
| Psychological | Baseline | |
| Hospital Anxiety and Depression Scale score | Pre-op (post-prehab) | |
| 3-month follow-up | ||
| Quality of life (health-related) | Baseline | |
| EuroQol-5 dimension | 3-month follow-up | |
| Others | ||
| Acute post-op hospital length-of-stay | During admission for surgery | |
| Post-op major complications rates (30 day) | During admission for surgery up to 30 days post-op | |
| Readmission rates (30 day & 3 mo) | Up to 3 months post-op | |
| Mortality (30 day & 3 mo) | Up to 3 months post-op | |
| Patient satisfaction survey to Prehab Program (see Appendix 3) | Pre-op (post-prehab) | |
| 3-month follow-up | ||
Table 2.
| Baseline characteristic | Value |
|---|---|
| Total screened | 211 (100) |
| Total enrolled | 182 (86.3) |
| Cancer types | |
| Colorectal | 120 (65.9) |
| Hepato-pancreato-biliary | 45 (24.7) |
| Urological | 13 (7.1) |
| Upper gastrointestinal | 4 (2.2) |
| Age (yr) | 70.43±10.64 |
| Sex, male | 108 (59.3) |
| Race | |
| Chinese | 143 (78.6) |
| Malay | 25 (13.7) |
| Indian | 9 (4.9) |
| Others | 5 (2.8) |
| Frailty score | |
| Frail | 92 (50.5) |
| Pre-frail | 48 (26.4) |
| Non-frail | 42 (23.1) |
| Smoking | |
| Non-smokers | 147 (80.8) |
| Current smokers | 15 (8.2) |
| Ex-smokers | 20 (11.0) |
| Alcohol | |
| Current significant intake | 9 (4.9) |
| Duration of prehabilitation (day) | 19.29±14.87 |
| Compliance | |
| Reported doing at least the minimum sets prescribed | 61 (65.6)a) |
| Able to demonstrate all exercises correctly during pre-operative follow-up | 81 (65.9)b) |
| Source of referrals | |
| Inpatient | 57 (31.3) |
| Outpatient | 125 (68.7) |
Table 3.
| Outcome measure | Baseline | Pre-operative | Improvement | p-valuea) | |
|---|---|---|---|---|---|
| Functional (physical) | |||||
| 6-minute walk test (m) | 303.94 (285.66–322.22) | 325.46 (305.14–345.77) | 21.52 | <0.001 | |
| 308 (234, 365) | 326 (251, 402) | ||||
| 30-Second Sit to Stand test (reps) | 10.99 (10.23–11.76) | 12.07 (11.25–12.90) | 1.08 | <0.001 | |
| 10 (9, 13) | 11 (9, 14) | ||||
| Timed Up and Go test (s) | 12.07 (10.87–13.27) | 11.24 (10.18–12.29) | 0.83 | 0.014 | |
| 10.9 (8.4, 14.8) | 9.5 (8.0, 12.4) | ||||
| Psychological (score) | |||||
| HADS depression | 2.93 (2.41–3.46) | 1.94 (1.46–2.43) | 0.99 points (34%) | <0.001 | |
| HADS anxiety | 3.24 (2.63–3.86) | 2.53 (1.93–3.12) | 0.71 points (22%) | 0.027 | |
| HADS total | 6.17 (5.17–7.16) | 4.40 (3.42–5.37) | 1.77 points (29%) | <0.001 | |
| Health-related quality-of-life | |||||
| EuroQol-5 dimension health score | 69.32 (65.96–72.68) | 76.36 (72.42–80.29)b) | 7.04 points | 0.001 | |
Table 4.
| Outcome measure | Pre-operative | Post-operative | Difference | p-valuea) | |
|---|---|---|---|---|---|
| Functional (physical) | |||||
| 6-minute walk test (m) | 305.94 (280.14–331.74) | 306.16 (280.39–331.92) | 0.22 | 0.964 | |
| 30-Second Sit to Stand test (reps) | 11.35 (10.29–12.42) | 11.43 (10.34–12.51) | 0.08 | 0.863 | |
| Timed Up and Go test (s) | 12.24 (10.77–13.72) | 12.28 (10.96–13.59) | 0.04 | 0.939 | |
| Psychological (score) | |||||
| HADS depression | 2.31 (1.61–3.01) | 1.79 (1.22–2.36) | 0.52 points (23%) | 0.117 | |
| HADS anxiety | 2.82 (1.99–3.65) | 1.46 (0.83–2.09) | 1.36 points (48%) | 0.001 | |
| HADS total | 4.40 (3.42–5.38) | 2.34 (1.18–3.49) | 2.06 points (47%) | 0.003 | |
Table 5.
Appendices
Appendix 1.
Prehabilitation protocol. Prehab, prehabilitation; PC, done by prehab coordinator; HADS, Hospital Anxiety and Depression Scale; EQ-5D, EuroQol-5 dimension; Physiatrist, done by physiatrist; CVM, cardiology; Ortho, orthopaedics surgery; BMI, body mass index; BADLs, Basic Activities of Daily Living.
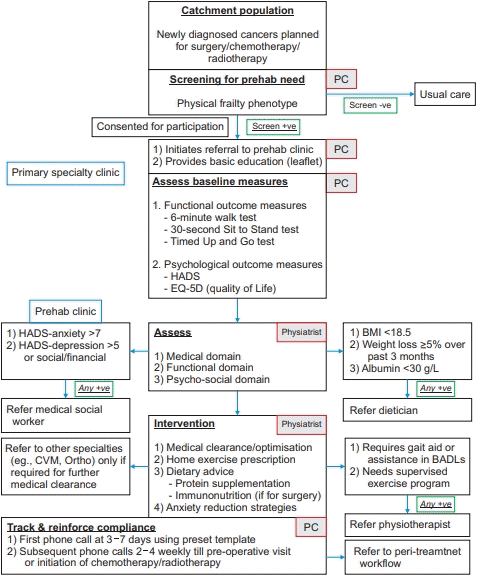
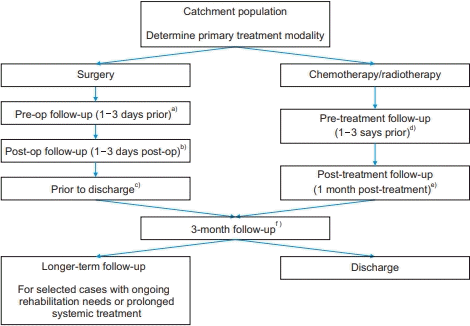
Appendix 2.
Peri-treatment workflow. Op, operative; PC, prehabilitation coordinator; HADS, Hospital Anxiety and Depression Scale; Prehab, prehabilitation; EQ-5D, EuroQol-5 dimension.
a. Siting for further rehabilitation ( inpatient rehabilitation vs. outpatient vs. home)
b. Medical optimization
c. Reinforcement/adjustment of exercise prescription
a. Referral/communication to inpatient dietician
b. Referral/communication to inpatient physiotherapist
c. Referral to inpatient Rehab Team (only if required)
a. Appointments with Prehab Clinic (physiatrist/PC) in 3 months
b. Outpatient referral to physiotherapist/occupational therapist (only if required, after discussion with physiatrist)
a. Need for outpatient physiotherapy/occupational therapy referral (eg., in cases of functional decline)
b. Medical optimization
c. Nutritional assessment & need for outpatient dietician referral
d. Mental health assessment & need for further referral to medical social worker
e. Reinforcement/adjustment of exercise prescription




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download