Abstract
Background
Methods
Results
Supplementary material
Supplementary Fig 1.
Notes
AUTHOR CONTRIBUTIONS
Conception or design: J.Y.J., S.J.H. Acquisition, analysis, or interpretation of data: J.Y.J., S.H.B., B.H.P., S.J.H. Drafting the work or revising: J.Y.J., N.L., H.J.K., D.J.K., K.W.L., S.J.H. Final approval of the manuscript: J.Y.J., S.H.B., B.H.P., N.L., H.J.K., D.J.K., K.W.L., S.J.H.
ACKNOWLEDGMENTS
REFERENCES
Fig. 1.
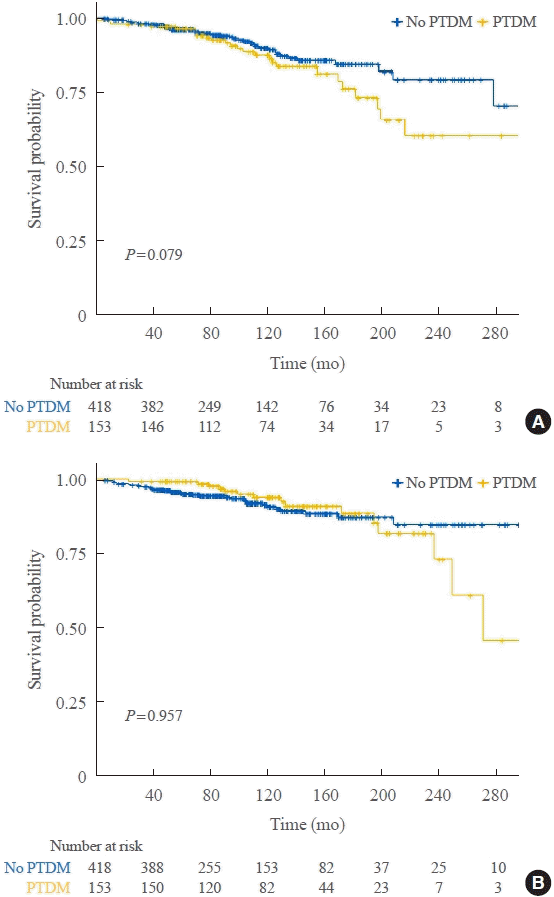
Table 1.
| Characteristic | No PTDM (n = 418) | PTDM (n = 153) | P value | |
|---|---|---|---|---|
| Age, yr | 42.6 ± 10.4 | 46.4 ± 9.0 | < 0.001 | |
| Male sex | 236 (56.5) | 82 (53.6) | 0.569 | |
| BMI, kg/m2 | 21.8 (20–24) | 23.0 (21–25) | < 0.001 | |
| Glucose, mg/dLa | 93.3 ± 12.2 | 96.5 ± 12.4 | 0.007 | |
| HbA1c, %b | 5.16 ± 0.42 | 5.30 ± 0.54 | 0.023 | |
| Type of dialysis | ||||
| Peritoneal dialysis | 67 (16.0) | 24 (15.7) | 0.936 | |
| Hemodialysis | 347 (83.0) | 128 (83.7) | ||
| Preemptive | 4 (1.0) | 1 (0.7) | ||
| Smoking | 22 (13.2) | 48 (31.4) | < 0.001 | |
| Comorbidities | ||||
| HTN | 271 (64.8) | 125 (81.7) | < 0.001 | |
| CVD | 12 (2.9) | 6 (3.9) | 0.589 | |
| Waiting time, mo | 35.7 (5–70) | 34.5 (3–66) | 0.708 | |
| Immunosuppressive drugs | ||||
| Tacrolimus | 242 (57.9) | 102 (66.7) | 0.067 | |
| Cyclosporine A | 176 (42.1) | 51 (33.3) | ||
| Cause of ESRD | ||||
| GNc | 103 (24.6) | 20 (13.1) | 0.049 | |
| Hypertension | 15 (3.6) | 6 (3.9) | ||
| Lupus | 7 (1.7) | 4 (2.6) | ||
| PCKD | 13 (3.1) | 9 (5.9) | ||
| Miscellaneousd | 5 (1.2) | 1 (0.7) | ||
| Unknown | 275 (65.8) | 113 (73.9) | ||
| Donor characteristics | ||||
| Age, yr | 42.7 (31–52) | 43.4 (30–51) | 0.893 | |
| Male sex | 249 (59.6) | 62 (40.5) | 0.999 | |
| ABO incompatible | 7 (1.7) | 3 (2.0) | 0.730 | |
| Living | 208 (49.9) | 85 (56.3) | 0.185 | |
| Transplant era | ||||
| 1994–2007 | 116 (27.8) | 46 (30.1) | 0.601 | |
| 2008–2017 | 302 (76.5) | 107 (69.9) | ||
Values are expressed as mean±standard deviation, number (%), or median (interquartile range).
PTDM, post-transplant diabetes mellitus; BMI, body mass index; HbA1c, hemoglobin A1c; HTN, hypertension; CVD, cardiovascular disease; ESRD, end-stage renal disease; GN, glomerulonephritis; PCKD, polycystic kidney disease.
Table 2.
| Variable | Hazard ratio |
95% CI |
P value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| All-cause mortalitya | |||||
| Age (per year increase) | 1.064 | 1.027 | 1.102 | < 0.001 | |
| Female sex | 1.881 | 0.948 | 3.733 | 0.071 | |
| Smoker | 2.330 | 0.964 | 5.631 | 0.060 | |
| BMI, kg/m2 (per 1 kg/m2 increase) | 1.055 | 0.946 | 1.177 | 0.338 | |
| Hypertension | 2.002 | 0.683 | 5.870 | 0.206 | |
| History of cardiovascular disease | 1.175 | 0.268 | 5.150 | 0.831 | |
| 2008–2017 transplant era (vs. 1994–2007) | 0.351 | 0.165 | 0.747 | 0.007 | |
| Donor age | 1.012 | 0.988 | 1.036 | 0.343 | |
| Deceased donor (vs. living donor) | 1.830 | 0.950 | 3.523 | 0.071 | |
| PTDM | 3.722 | 0.658 | 21.042 | 0.137 | |
| Duration of PTDM | 1.239 | 0.933 | 1.646 | 0.139 | |
| Four-point major adverse cardiovascular eventsb | |||||
| Age (per year increase) | 1.048 | 1.016 | 1.081 | 0.003 | |
| Female sex | 0.902 | 0.492 | 1.654 | 0.738 | |
| Smoker | 2.247 | 1.150 | 4.389 | 0.018 | |
| BMI, kg/m2 (per 1 kg/m2 increase) | 1.050 | 0.949 | 1.163 | 0.344 | |
| Hypertension | 1.411 | 0.765 | 2.603 | 0.271 | |
| History of cardiovascular disease | 5.091 | 2.255 | 11.494 | < 0.001 | |
| 2008–2017 transplant era (vs. 1994–2007) | 0.566 | 0.293 | 1.094 | 0.090 | |
| Donor age | 1.009 | 0.989 | 1.029 | 0.370 | |
| Deceased donor (vs. living donor) | 3.412 | 1.838 | 6.335 | < 0.001 | |
| PTDM | 0.600 | 0.284 | 1.268 | 0.181 | |
| Duration of PTDM | 1.101 | 1.024 | 1.183 | 0.009 | |
Table 3.
| Variable | Hazard ratio |
95% CI |
P value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Overall graft failurea | |||||
| Age (per year increase) | 0.990 | 0.970 | 1.009 | 0.290 | |
| Female sex | 1.335 | 0.912 | 1.957 | 0.138 | |
| Smoker | 1.546 | 0.951 | 2.516 | 0.079 | |
| BMI, kg/m2 (per 1 kg/m2 increase) | 1.053 | 0.988 | 1.123 | 0.113 | |
| Hypertension | 1.655 | 0.888 | 3.084 | 0.113 | |
| History of cardiovascular disease | 2.041 | 0.923 | 4.513 | 0.078 | |
| 2008–2017 transplant era (vs. 1994–2007) | 0.640 | 0.415 | 0.988 | 0.044 | |
| Donor age | 1.028 | 1.014 | 1.043 | < 0.001 | |
| Deceased donor (vs. living donor) | 1.480 | 1.008 | 2.173 | 0.046 | |
| PTDM | 2.331 | 0.945 | 5.520 | 0.054 | |
| Duration of PTDM | 1.121 | 0.985 | 1.276 | 0.084 | |
| Death-censored graft failureb | |||||
| Age (per year increase) | 0.965 | 0.944 | 0.987 | 0.002 | |
| Female sex | 1.195 | 0.762 | 1.874 | 0.439 | |
| Smoker | 1.566 | 0.905 | 2.710 | 0.109 | |
| BMI, kg/m2 (per 1 kg/m2 increase) | 1.053 | 0.977 | 1.134 | 0.177 | |
| Hypertension | 1.798 | 1.110 | 2.914 | 0.017 | |
| History of cardiovascular | 2.686 | 1.124 | 6.422 | 0.026 | |
| 2008–2017 transplant era (vs. 1994–2007) | 0.754 | 0.455 | 1.249 | 0.273 | |
| Donor age | 1.034 | 1.017 | 1.051 | < 0.001 | |
| Deceased donor (vs. living donor) | 1.436 | 0.915 | 2.253 | 0.116 | |
| PTDM | 2.109 | 0.783 | 5.684 | 0.140 | |
| Duration of PTDM | 1.111 | 0.961 | 1.285 | 0.154 | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download