Abstract
There are several controversies regarding the role of sex and gender in the pathophysiology and management of acute stroke. Assessing the role of sex, i.e., biological/pathophysiological factors, and gender, i.e., sociocultural factors, in isolation is often not possible since they are closely intertwined with each other. To complicate matters even more, the functional baseline status of women and men at the time of their first stroke is substantially different, whereby women have, on average, a poorer reported/ascertained baseline function compared to men. These differences in baseline variables account for a large part of the differences in post-stroke outcomes between women and men. Adjusting for these baseline differences is difficult, and in many cases, residual confounding cannot be excluded. Despite these obstacles, a better understanding of how patient sex and gender differences influence acute stroke and stroke care pathways is crucial to avoid biases and allow us to provide the best possible care for all acute stroke patients. Disregarding patient sex and gender on one hand and ignoring potential confounding factors in sex- and gender-stratified analyses on the other hand, may cause researchers to come to erroneous conclusions and physicians to provide suboptimal care. This review outlines sex- and gender-related factors in key aspects of acute stroke, including acute stroke epidemiology, diagnosis, access to care, treatment outcomes, and post-acute care. We also attempt to outline knowledge gaps, which deserve to be studied in further detail, and practical implications for physicians treating acute stroke patients in their daily practice.
Sex and gender differences are a complex topic across medicine, and acute stroke is no exception. Several important differences among patients with acute stroke are related to either biology/pathophysiology, or sociocultural factors, such as education, power, and financial status. The former are considered sex-specific differences, and in humans, sex is classified as either male or female and considered static, i.e., it does not change over a person’s lifetime [1]. The latter, on the other hand, are considered gender-specific differences, whereby gender refers to social roles and behaviors [1]. Besides gender identity, gender also encompasses gender relations and roles. As opposed to sex, gender is neither binary nor static [2]: It exists along a continuum and may change over time.
Disentangling whether certain differences between women and men are related to sex or gender is often challenging, if not impossible. Doing so is, however, important because it can provide us with valuable insights into how acute stroke treatment for a particular sex or gender can be improved. Modifying sex-related factors, which are biological variables or pathophysiological pathways, often requires medical/pharmacological treatment, and in some instances, sex-related factors may not be modifiable at all (e.g., chromosomal differences between males and females). Gender-related factors on the other hand, are in theory modifiable without any pharmacological intervention, but in practice, the sociocultural environment of the patients may not allow for changes. Examples for such sociocultural factors that are in principle modifiable but in practice hard to change include education level, financial status, and caregiver responsibilities.
Assessing the role of gender-related factors in acute stroke care is further complicated by intersectionality, i.e., the interconnectedness of patients’ gender, functional status, age, ethnicity, financial status, and social status, which are tightly related to each other in a complex relationship, but often treated as independent, unrelated variables in our research [3]. The intersectionality concept is of particular importance when assessing the role of gender in acute stroke: for example, it is likely that under-representation of females in a study sample is also associated with under-and misrepresentation of elderly and pre-stroke disabled patients [4].
While biological, sex-related factors that influence acute stroke care may be assumed to have similar importance across different sociocultural settings, the importance of gender-related, sociocultural factors may vary largely in different sociocultural settings, although data on this topic are currently still lacking for the most part. Because acute stroke researchers usually do not explicitly assess patients’ sociocultural roles and gender identity, there is mostly no clear distinction between sex and gender in acute stroke research, and the vast majority of studies have focused on sex differences. We will therefore refer to “patient sex” and “sex-related differences” in the remainder of the manuscript unless specifically referring to gender-related aspects, being well aware of the fact that some of the presumed sex-related phenomena may in fact truly be gender-related.
In the following, we attempt to summarize sex and gender differences with regard to key aspects of acute stroke, including stroke epidemiology, diagnosis, access to treatment, technical and clinical outcomes after intravenous thrombolysis (IVT) and endovascular treatment (EVT), and early post-acute care. We also attempt to outline knowledge gaps, which, in our opinion, deserve to be studied in further detail, and concrete, practical implications for practicing physicians. Of note, while we acknowledge that acute stroke encompasses hemorrhagic stroke types as well (that is, intracranial hemorrhage and subarachnoid hemorrhage), we will focus on acute ischemic stroke (AIS) in this review.
There is a complicated relationship between the stroke incidence and mortality, age, and sex. To begin with, the sex differences in stroke incidence and mortality vary by age: in young patients (below 45 years of age), stroke incidence and mortality are similar in both sexes. Between age 45 and 74 years, males show higher incidence and mortality, while above 74 years, the reverse is true [5]. Of note, since female’s life expectancy exceeds the one of males, there is a preponderance of females among the very elderly, and thus, the disadvantage of females in the very old age groups overall “outweighs” the effects in the young and middle-aged groups. As a result, in the United States (US), the proportion of deaths caused by stroke in females is higher than in males [6]. In the year 2019, stroke was the third leading cause of death in females in the US, accounting for 6.2% of all female deaths, and the fifth-leading cause of death in males, accounting for 4.4% of all male deaths. 7 Fortunately, over the last years, stroke incidence has declined in the US, at a similar pace in both sexes [8].
Baseline status of women and men at the time of their first stroke is substantially different, whereby women have, on average, a poorer functional baseline status compared to men.
They are on average older [9], have more comorbidities, have higher pre-stroke disability [10], and live alone more often [11-14]. There are also important sex-related differences with regard to stroke etiology and stroke subtypes: females suffer more often from atrial fibrillation and subsequent cardioembolic strokes [15], probably resulting in more large vessel occlusion strokes. Males more commonly smoke and drink on average more alcohol [16], which are risk factors for atherosclerosis and subsequent atherosclerotic strokes. Figure 1 and Table 1 provide an overview of sex- and gender-specific differences in baseline variables among AIS patients. Multivariable analysis can in theory partially address this problem, but some residual confounding almost always remains because not all the relevant factors get captured. For example, it may well be that the poorer pre-stroke cognitive function of women substantially contributes to their poorer post-stroke outcomes, but this hypothesis is hard to prove, since, from a practical standpoint, it is very hard to capture the pre-stroke cognitive status in stroke patients. These “unknown” baseline differences make head-to-head comparisons among women and men difficult [17]. Some may even wonder whether the on average poorer post-stroke outcomes in women compared to men can be entirely explained by differences in baseline status. However, there are conflicting data on whether this is truly the case [10,18] as even after controlling for known differences in baseline variables, women continue to have poorer functional outcomes after stroke compared to men, at a magnitude that may not necessarily be fully explained by unknown differences in baseline variables. One additional problem in this regard is that there may be gender-related biases in reporting of outcomes and variables such as baseline functional status, i.e., the tendency of patients to over- or under-estimate their abilities may partly depend on their gender.
For obvious reasons, most clinical acute stroke studies include only patients that have managed to get in contact with stroke systems of care during the acute phase of their stroke. This means that there is a “denominator problem,” i.e., we do not know how many patients that are in need of acute stroke care have not received care, and whether the proportions of these patients are different in women vs. men (Figure 2). It is also likely that these discrepancies differ between sociocultural environments and various regions of the world. On a global level, imbalances in power and education as well as financial dependency of women on their male family members lead to restricted access to education, including health education, and healthcare [19]. This most likely negatively affects access to acute stroke care as well. For example, the willingness to pay for acute stroke care for female family members may be lower compared to male family members in some countries, since the overall family structure is heavily male-focused and women often formally do not contribute to the family income. In addition, due to their lower education level, including health education, females may have difficulties recognizing themselves that they suffer from an acute stroke. Furthermore, in some settings, female healthcare staff are restricted in their practice or prevented from practicing altogether, which constitutes an additional sociocultural barrier for women to seek medical care [20]. All these points are however mostly speculative at this point because robust data, especially from non-industry nations, are largely missing. Due to the aforementioned denominator problem, published data can only help us to indirectly infer sex- and gender-related disparities in access to acute stroke care. For example, in two large Middle Eastern registries, male stroke patients accounted for almost 70% of the study sample, a gender imbalance that is not plausible when considering biological factors alone. We may therefore suspect that lacking access of women to acute stroke care is the reason for this discrepancy, but we cannot prove this based on currently available data [21,22]. Studies from Western countries also show longer delays in the pre-hospital phase of female AIS patients compared to men, partly because of prolonged times between symptom onset and call for help, which in turn, may be explained by women living alone more often compared to men [11,13,14,23].
Traditionally, it has been thought that acute stroke is more often under- and misdiagnosed in women because they frequently present with atypical stroke symptoms. Whether atypical stroke symptoms are truly more common in women than in men is however controversial, since most studies on the topic were not adjusted for potential confounders such as age and concomitant psychiatric and psychosomatic disorders [24]. In a large prospective Canadian cohort study, women were more likely to be diagnosed with stroke mimics although their symptoms did not differ from those of men, and their risk of subsequent vascular events was also similar to the risk of men [25]. What is well-known is that the proportion of suspected acute stroke patients that in fact suffer from stroke mimics, particularly migraine, is higher among females [24,26,27], which poses a challenge to physicians, who have to distinguish between acute stroke and stroke mimics to initiate the appropriate treatment within a short time period. Indeed, in another prospective Canadian cohort study, revision of stroke mimic diagnoses to a final diagnosis of cerebrovascular events was common. However, women with a stroke mimic diagnosis in whom a suspected minor cerebrovascular event was initially expected had their stroke mimic diagnosis revised less frequently compared to men [27]. Once the diagnosis of acute stroke has been made, women are less likely to undergo diagnostic imaging, but this difference is almost certainly purely an age effect, whereby women are on average older, and the sex differences disappear after adjusting for patient age. Time to imaging is however longer in women, even after adjusting for age [24].
There are differences in IVT usage between women and men in some regions of the world. According to a large meta-analysis, women are less likely to receive IVT compared to men in the US and most European countries, but not in Asia and Germany, although the gap seems to diminish over time [28]. Subgroup analyses including only patients deemed eligible for IVT however did not show any significant sex difference in IVT treatment anymore [28]. Thus, one may assume that the sex-based IVT gap could largely be explained by differences in baseline characteristics; i.e., women are probably less often eligible for IVT, because they suffer more often from cardioembolic strokes that lead to larger infarcts, and thus, large established infarcts at baseline imaging may preclude IVT more often in women than in men. One caveat with regard to this hypothesis is that the criteria based on which IVT eligibility was judged are only vaguely stated or completely missing in many studies. It has also been hypothesized that women are less likely to agree to IVT. However, data in this regard are conflicting, with some, but not all studies showing a lower likelihood of women accepting IVT [29,30]. With regard to IVT workflow times, European studies showed similar workflow efficiency in both sexes, while North American data suggest slight IVT delays in women [24]. Of note, under-utilization and delay in IVT in women are more pronounced in hospitals without specialized stroke unit care [31]. As for treatment complications, it is possible that men may have a higher risk of intracranial hemorrhage following IVT [32]. As far as functional outcomes post-IVT are concerned, a few publications describe worse outcomes in women even after adjustment for baseline variables [33]. However, the majority of studies and particularly post-hoc analyses of large randomized controlled trials suggest that IVT is equally effective in both women and men [24]. Nevertheless, these post-hoc analyses need to be interpreted with caution: sex-stratified analyses were not pre-specified; as such, the trials were not adequately powered to detect sex-specific differences, and the inclusion criteria of IVT trials and clinical stroke trials in general, often restrict enrolment to patients below a certain age limit and exclude patients with substantial pre-existing disability. This disproportionately affects women and may lead to both under- and misrepresentation of women in these trials (Figure 3). In other words, the women included in clinical stroke trials may not be representative of the overall female stroke population. Although in principle, this applies to men too, it is less of a problem since they are on average younger and less disabled at the time of their first stroke.
Data regarding sex-specific differences in EVT eligibility and usage are highly heterogeneous: some studies from the US show similar rates of EVT among men and women [34], while data from Denmark and Japan suggest lower EVT rates in women [11,13]. Another US study [35] and a nationwide German study [36] on the other hand showed higher EVT utilization in women. One potential reason for increased EVT use in women could be the higher incidence of atrial fibrillation and cardio-embolic strokes in women, which more often results in large vessel occlusion compared to other stroke etiologies, while the decreased use compared to men in some studies could be a result of more “unfavorable” baseline characteristics of women, namely that they live alone more often, are on average older and more often disabled prior to their stroke. For non-industrial countries, there is relative paucity of data regarding sex-specific EVT use. In one Japanese registry study, door-to-arterial puncture times were longer in women [13]. Technical EVT success, that is recanalization of the occluded blood vessel, is similar in female and male patients [37,38]. With regard to clinical outcomes, post-hoc analyses of large randomized EVT trials [39] and large prospective registry studies [40] showed overall no sex-specific differences in 90-day outcomes and no differences in treatment effect after adjustment for confounding factors, but women may have greater gain in disability-free life [37,39]. Of note, the same limitations that were mentioned in the IVT paragraph apply, since all EVT trials had rather stringent inclusion criteria with pre-stroke disability caps, and most of them did not pre-specify sex-stratified analysis and were thus not adequately powered to detect sex-specific differences (Figure 3).
In the post-acute phase, given that the overall prognosis in female stroke patients is on average poorer, they are probably at greater risk of “withdrawal bias” than their male counterparts: treatment decisions may be biased by higher pre-stroke disability, possibly leading to delayed treatment and undertreatment, pre-mature withdrawal of care, and thus result in even poorer outcomes (“self-fulfilling prophecy”) [41]. Indeed, data from Western countries suggest that, despite their on average higher stroke severity, females have shorter hospital length of stay than males [14], and receive palliative care consultation more often [36] while being offered acute rehabilitation services less often [12,14,20].
Table 2 summarizes the above paragraphs and provides an overview of the discussed sex- and gender-related aspects in acute stroke. But what are the implications for stroke researchers and physicians treating acute stroke patients in their daily practice?
It is important to be aware of the overall poorer baseline functional status of women compared to men. For practicing physicians, this means that they should be cautious to not prematurely enter the “withdrawal of care cycle,” which may get triggered by poor baseline function (i.e., poor baseline status biases treatment decisions and leads to premature withdrawal of care, which in turn results in worse outcomes). They may want to decide to postpone the decision to proceed with palliative care in the first 24–72 hours as it may become a self-fulfilling prophecy [20]. For researchers, it is critical to remember that most sex- and gender-related differences in almost all aspects of acute stroke care are in fact the result of confounding by baseline status. Thus, adjusting for these variables is critical when trying to assess for “true” sex-/gender-related differences.
There is a large unmet need for robust evidence regarding sex- and gender-related discrepancies in access to acute stroke care. Determining sex- and gender-specific “access gaps” will be challenging, because it requires determining the “denominator,” i.e., the number of all patients that are in need of acute stroke care but do not get in contact with the medical system. Doing so is however crucial for researchers in order to estimate sex-/gender-specific access gaps and investigate the reasons for these gaps, for clinicians to enable them to actively encourage those who lack adequate access and their families to seek medical care, and for policymakers to address access gaps with stroke awareness campaigns, infrastructural changes, or other changes in the health-care system [20]. Comparing the sex/gender distribution of patients presenting to the hospital vs. out-of-hospital stroke deaths would be a starting point to estimate the size of the “denominator” population and could serve as an indicator of missed opportunities for acute care.
The increased incidence of stroke mimics, and particularly migraine, among women is probably the most obvious sex-/gender-related difference in stroke-like presentations. For clinicians, this means that they have to face a diagnostic challenge particularly in female patients. Since the benefit of acute stroke treatment is highly time-dependent [42], we would advise physicians to proceed with an acute stroke imaging protocol, including vascular imaging to assess for EVT-amenable vessel occlusions, as soon as possible if there is even the slightest doubt in a patient presumably suffering from a stroke mimic. Treatment for none of the stroke mimics is as time-dependent, so there is little harm in rapid image acquisition, while the potential downstream benefits are tremendous. From a research perspective, it would be desirable to investigate which diagnostic pathways, including imaging such as magnetic resonance imaging [25,27], could differentiate females with acute stroke vs. those with stroke mimics quickly and reliably, knowing that there will never be a perfect way to tell them apart.
The reasons why women are less likely to receive IVT are not yet fully understood. Factors that are likely to play a role are reduced eligibility among female patients due to poorer baseline status, possibly a more risk-averse behavior among females, and longer delays from onset to hospital arrival. Clinicians could try to partially address the latter problem pre-emptively, by explaining the signs and symptoms of stroke to patients with high stroke risk, especially women who are living alone, creating an action plan about how to activate emergency medical services, and discussing the option of having a life alert bracelet [20]. Researchers may want to explore the factors that lead to reduced IVT utilization in women in greater detail, since this may provide starting points to identify and, in a second step, reduce underutilization of IVT.
There is substantial heterogeneity in the literature with regard to sex-specific EVT use. Once EVT has been performed, overall outcomes in women are worse, again largely related to confounding baseline factors, but their gain in disability-free life is greater compared to male patients. Thus, clinicians may want to find out as many details as possible about what their patients consider to be a desirable outcome, from communication with the patients themselves and their family members, and center their treatment decisions around this goal. Our patients may consider outcomes as acceptable which we as physicians think of as undesirable, and this becomes particularly relevant when the overall prognosis is poor, as is the case in many female stroke patients. As researchers, we should strive towards investigating sex-specific differences in EVT utilization in more depth, and assess patient preferences with regard to clinical outcomes in greater detail, to allow for more informed treatment decisions in patients with poor overall prognosis.
There is robust evidence to suggest that women receive less defect-free care in the post-acute phase and are more likely to enter a palliative care pathway. Whether this is mostly due to withdrawal bias, poorer baseline status, or whether there are other contributing factors is currently unclear. As physicians, we should again be careful to not decide upon a palliative treatment regimen prematurely. From a research standpoint, a detailed investigation of the factors leading to sex- and gender-specific differences in post-acute stroke care, and particularly the role of withdrawal bias, would be desirable.
Patient sex and gender profoundly influence stroke risk, access to acute stroke care, and perhaps also the use of acute stroke therapies. Differences in post-stroke outcomes on the other hand are largely related to baseline confounding factors. Of note, based on all available evidence, both IVT and EVT are equally effective in both sexes. For physicians who treat acute stroke patients, it is important to be aware of sex- and gender-related differences in their daily clinical practice in order to provide the best possible care for patients of both sexes. Similarly, stroke researchers need to be aware of the impact of sex and gender in their data, and keep in mind the possibility of confounding through baseline factors, to avoid erroneous conclusions. Doing so will further enhance our understanding of the role sex and gender play in acute stroke care, and help to improve outcomes of all patients suffering from acute stroke.
References
1. Moleiro C, Pinto N. Sexual orientation and gender identity: review of concepts, controversies and their relation to psychopathology classification systems. Front Psychol. 2015; 6:1511.
2. Day S, Mason R, Lagosky S, Rochon PA. Integrating and evaluating sex and gender in health research. Health Res Policy Syst. 2016; 14:75.
3. Labovitz DL. Stroke epidemiology and intersectionality. Stroke. 2020; 51:2886–2887.
4. Ganesh A, Fraser JF, Gordon Perue GL, Amin-Hanjani S, Leslie-Mazwi TM, Greenberg SM, et al. Endovascular treatment and thrombolysis for acute ischemic stroke in patients with premorbid disability or dementia: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 2022; 53:e204–e217.
5. Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008; 7:915–926.
6. Rexrode KM, Madsen TE, Yu AYX, Carcel C, Lichtman JH, Miller EC. The impact of sex and gender on stroke. Circ Res. 2022; 130:512–528.
7. Heron M. Deaths: leading causes for 2019. Natl Vital Stat Rep. 2021; 70:1–114.
8. Koton S, Sang Y, Schneider ALC, Rosamond WD, Gottesman RF, Coresh J. Trends in stroke incidence rates in older US adults: an update from the Atherosclerosis Risk in Communities (ARIC) cohort study. JAMA Neurol. 2020; 77:109–113.
9. Appelros P, Stegmayr B, Terént A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009; 40:1082–1090.
10. Persky RW, Turtzo LC, McCullough LD. Stroke in women: disparities and outcomes. Curr Cardiol Rep. 2010; 12:6–13.
11. Mainz J, Andersen G, Valentin JB, Gude MF, Johnsen SP. Disentangling sex differences in use of reperfusion therapy in patients with acute ischemic stroke. Stroke. 2020; 51:2332–2338.
12. Madsen TE, DeCroce-Movson E, Hemendinger M, McTaggart RA, Yaghi S, Cutting S, et al. Sex differences in 90-day outcomes after mechanical thrombectomy for acute ischemic stroke. J Neurointerv Surg. 2019; 11:221–225.
13. Uchida K, Yoshimura S, Sakai N, Yamagami H, Morimoto T. Sex differences in management and outcomes of acute ischemic stroke with large vessel occlusion. Stroke. 2019; 50:1915–1918.
14. Willers C, Lekander I, Ekstrand E, Lilja M, Pessah-Rasmussen H, Sunnerhagen KS, et al. Sex as predictor for achieved health outcomes and received care in ischemic stroke and intracerebral hemorrhage: a register-based study. Biol Sex Differ. 2018; 9:11.
15. Niewada M, Kobayashi A, Sandercock PA, Kamin´ski B, Członkowska A; International Stroke Trial Collaborative Group. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology. 2005; 24:123–128.
16. Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke. 2003; 34:1581–1585.
17. Renoux C, Coulombe J, Li L, Ganesh A, Silver L, Rothwell PM; Oxford Vascular Study. Confounding by pre-morbid functional status in studies of apparent sex differences in severity and outcome of stroke. Stroke. 2017; 48:2731–2738.
18. Reeves MJ, Fonarow GC, Zhao X, Smith EE, Schwamm LH; Get With The Guidelines-Stroke Steering Committee & Investigators. Quality of care in women with ischemic stroke in the GWTG program. Stroke. 2009; 40:1127–1133.
19. World Medical Association. World Medical Association Statement on Access of Women and Children to Health Care [Internet]. Ferney-Voltaire, France: WMA;[cited 2022 Dec 18]. Available from: https://www.wma.net/policiespost/wma-resolution-on-access-of-women-and-childrento-health-care-and-the-role-of-women-in-the-medicalprofession/.
20. Ospel JM, Schaafsma JD, Leslie-Mazwi TM, Amin-Hanjani S, Asdaghi N, Gordon-Perue GL, et al. Toward a better understanding of sex-and gender-related differences in endovascular stroke treatment: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 2022; 53:e396–e406.
21. al-Rajeh S, Larbi EB, Bademosi O, Awada A, Yousef A, al-Freihi H, et al. Stroke register: experience from the eastern province of Saudi Arabia. Cerebrovasc Dis. 1998; 8:86–89.
22. Al-Rukn S, Mazya M, Akhtar N, Hashim H, Mansouri B, Faouzi B, et al. Stroke in the Middle-East and North Africa: a 2-year prospective observational study of intravenous thrombolysis treatment in the region. Results from the SITS-MENA registry. Int J Stroke. 2020; 15:980–987.
23. Foerch C, Misselwitz B, Humpich M, Steinmetz H, Neumann-Haefelin T, Sitzer M; Arbeitsgruppe Schlaganfall Hessen. Sex disparity in the access of elderly patients to acute stroke care. Stroke. 2007; 38:2123–2126.
24. Bushnell C, Howard VJ, Lisabeth L, Caso V, Gall S, Kleindorfer D, et al. Sex differences in the evaluation and treatment of acute ischaemic stroke. Lancet Neurol. 2018; 17:641–650.
25. Yu AYX, Penn AM, Lesperance ML, Croteau NS, Balshaw RF, Votova K, et al. Sex differences in presentation and outcome after an acute transient or minor neurologic event. JAMA Neurol. 2019; 76:962–968.
26. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002; 346:257–270.
27. Yu AYX, Hill MD, Asdaghi N, Boulanger JM, Camden MC, Campbell BCV, et al. Sex differences in diagnosis and diagnostic revision of suspected minor cerebral ischemic events. Neurology. 2021; 96:e732–e739.
28. Strong B, Lisabeth LD, Reeves M. Sex differences in IV thrombolysis treatment for acute ischemic stroke: a systematic review and meta-analysis. Neurology. 2020; 95:e11–e22.
29. Kapral MK, Devon J, Winter AL, Wang J, Peters A, Bondy SJ. Gender differences in stroke care decision-making. Med Care. 2006; 44:70–80.
30. Vahidy FS, Rahbar MH, Lal AP, Grotta JC, Savitz SI. Patient refusal of thrombolytic therapy for suspected acute ischemic stroke. Int J Stroke. 2015; 10:882–886.
31. Pérez-Sánchez S, Barragán-Prieto A, Ortega-Quintanilla J, Domínguez-Mayoral A, Gamero-García MÁ, Zapata-Arriaza E, et al. Sex differences by hospital-level in performance and outcomes of endovascular treatment for acute ischemic stroke. J Stroke. 2020; 22:258–261.
32. Lorenzano S, Ahmed N, Falcou A, Mikulik R, Tatlisumak T, Roffe C, et al. Does sex influence the response to intravenous thrombolysis in ischemic stroke?: answers from safe implementation of treatments in Stroke-International Stroke Thrombolysis Register. Stroke. 2013; 44:3401–3406.
33. Spaander FH, Zinkstok SM, Baharoglu IM, Gensicke H, Polymeris A, Traenka C, et al. Sex differences and functional outcome after intravenous thrombolysis. Stroke. 2017; 48:699–703.
34. Lekoubou A, Bishu KG, Ovbiagele B. Stroke thrombectomy utilization rates by sex: what were things like before 2015? J Stroke Cerebrovasc Dis. 2020; 29:104587.
35. Otite FO, Saini V, Sur NB, Patel S, Sharma R, Akano EO, et al. Ten-year trend in age, sex, and racial disparity in tPA (Alteplase) and thrombectomy use following stroke in the United States. Stroke. 2021; 52:2562–2570.
36. Weber R, Krogias C, Eyding J, Bartig D, Meves SH, Katsanos AH, et al. Age and sex differences in ischemic stroke treatment in a nationwide analysis of 1.11 million hospitalized cases. Stroke. 2019; 50:3494–3502.
37. Sheth SA, Lee S, Warach SJ, Gralla J, Jahan R, Goyal M, et al. Sex differences in outcome after endovascular stroke therapy for acute ischemic stroke. Stroke. 2019; 50:2420–2427.
38. Dmytriw AA, Ku JC, Yang VXD, Hui N, Uchida K, Morimoto T, et al. Do outcomes between women and men differ after endovascular thrombectomy? A meta-analysis. AJNR Am J Neuroradiol. 2021; 42:910–915.
39. Chalos V, de Ridder IR, Lingsma HF, Brown S, van Oostenbrugge RJ, Goyal M, et al. Does sex modify the effect of endovascular treatment for ischemic stroke? Stroke. 2019; 50:2413–2419.
40. Deb-Chatterji M, Schlemm E, Flottmann F, Meyer L, Alegiani A, Brekenfeld C, et al. Sex differences in outcome after thrombectomy for acute ischemic stroke are explained by confounding factors. Clin Neuroradiol. 2021; 31:1101–1109.
41. Holloway RG, Arnold RM, Creutzfeldt CJ, Lewis EF, Lutz BJ, McCann RM, et al. Palliative and end-of-life care in stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014; 45:1887–1916.
42. Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016; 316:1279–1288.
43. Gardener H, Sacco RL, Rundek T, Battistella V, Cheung YK, Elkind MSV. Race and ethnic disparities in stroke incidence in the Northern Manhattan study. Stroke. 2020; 51:1064–1069.
44. Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 2009; 40:1032–1037.
45. Peters SAE, Carcel C, Millett ERC, Woodward M. Sex differences in the association between major risk factors and the risk of stroke in the UK Biobank cohort study. Neurology. 2020; 95:e2715–e2726.
46. Peters SA, Huxley RR, Woodward M. Comparison of the sex-specific associations between systolic blood pressure and the risk of cardiovascular disease: a systematic review and meta-analysis of 124 cohort studies, including 1.2 million individuals. Stroke. 2013; 44:2394–2401.
47. Madsen TE, Howard G, Kleindorfer DO, Furie KL, Oparil S, Manson JE, et al. Sex differences in hypertension and stroke risk in the REGARDS study: a longitudinal cohort study. Hypertension. 2019; 74:749–755.
48. Demel SL, Kittner S, Ley SH, McDermott M, Rexrode KM. Stroke risk factors unique to women. Stroke. 2018; 49:518–523.
49. Li C, Hedblad B, Rosvall M, Buchwald F, Khan FA, Engström G. Stroke incidence, recurrence, and case-fatality in relation to socioeconomic position: a population-based study of middle-aged Swedish men and women. Stroke. 2008; 39:2191–2196.
Figure 1.
Illustration of key sex- and gender-related differences in stroke patients’ baseline status.
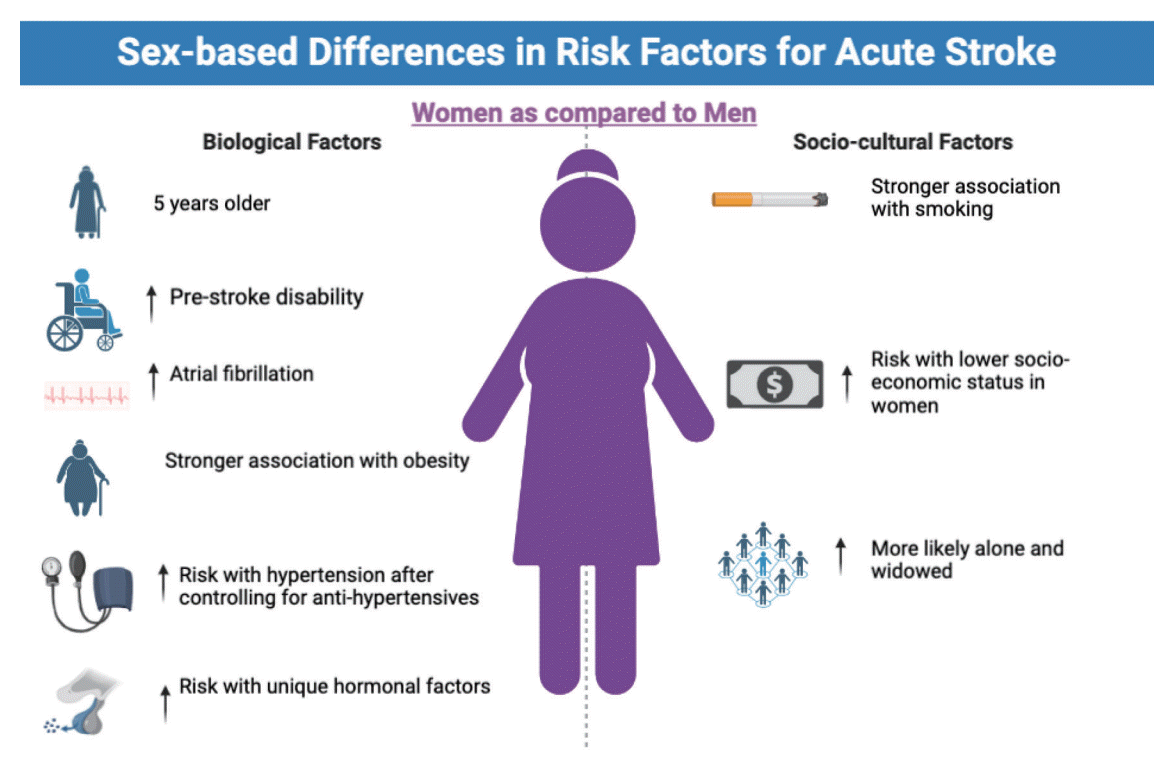
Figure 2.
Sex- and gender-related factors in endovascular stroke treatment and intravenous thrombolysis. The yellow box illustrates societal (gender-related) factors that influence the treatment decision. The blue box illustrates biological (sex-related) factors that influence the treatment decision. Note that the inter-relationship between these variables is complex, whereby various societal factors influence each other, and the same is true for biological factors. There are also inter-relationships between societal and biological factors, as indicated by the dashed arrows. Also, note that this illustration merely points out exemplary inter-relationships between these factors and is not providing a comprehensive summary of all possible interactions and inter-relationships. To come to a treatment decision, there are access barriers (can the patient make contact with the healthcare system?), diagnostic challenges (are the acute ischemic stroke [AIS] symptoms recognized as such, and the correct diagnosis is made?) and decision challenges (should treatment be initiated, given the patient’s baseline status?) need to be overcome before treatment with endovascular treatment (EVT) or intravenous thrombolysis (IVT) is initiated. Based on all available evidence, neither EVT nor IVT treatment effect is modified based on sex. If treatment is initiated, current evidence suggests that women will sustain overall more post-stroke disability. However, they also benefit from a greater gain in disability-free life years compared to men.
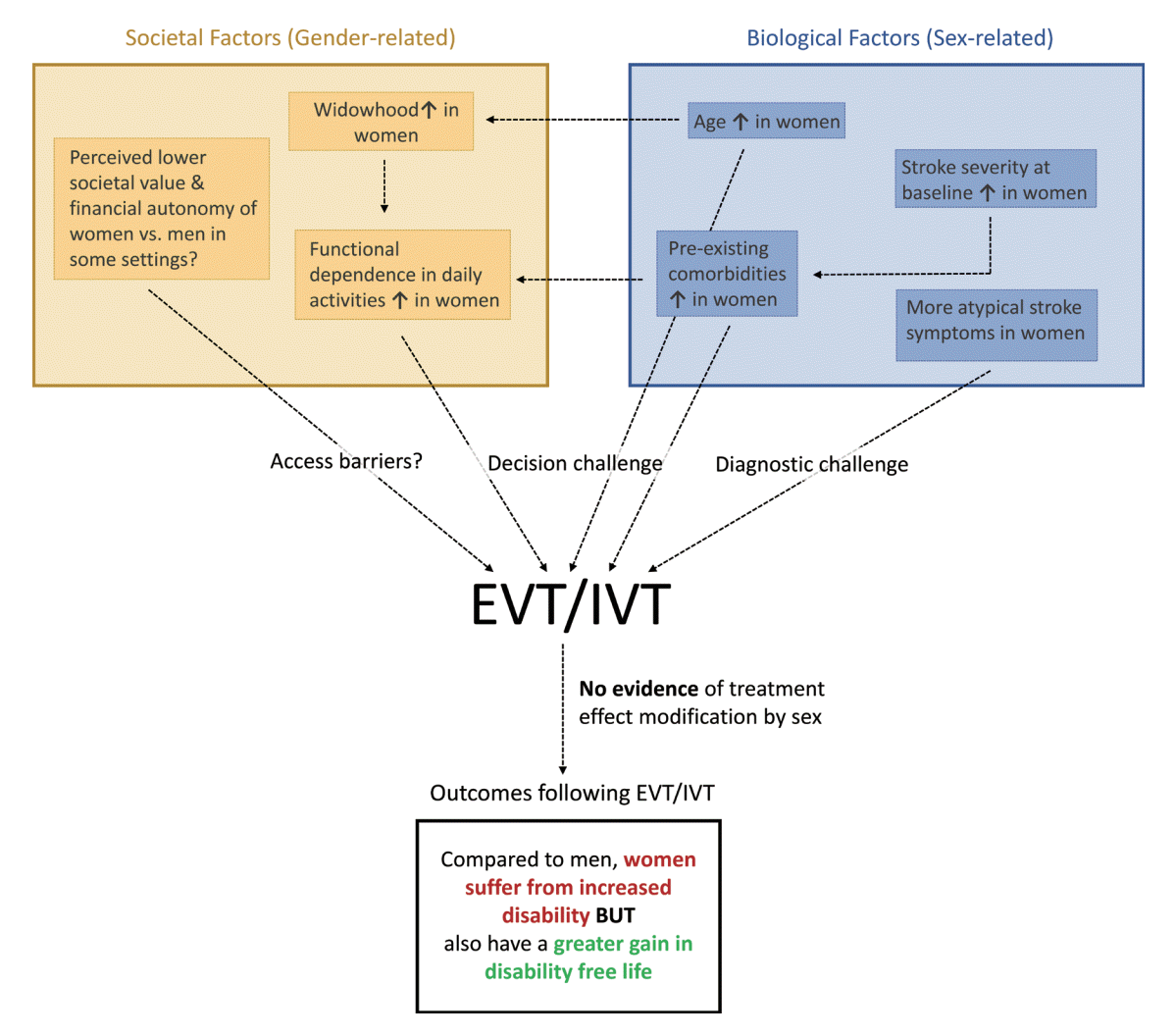
Figure 3.
Exclusion criteria in clinical trials and their effect on the representativeness of the female and male stroke patient population. The blue circle shows the entity of male stroke patients and the red circle shows the entity of female stroke patients on a population level. The x-axis indicates patient age at stroke onset, while the y-axis indicates pre-stroke disability at stroke onset. Since female stroke patients are on average older and suffer from more severe pre-stroke disability compared to males, applying an upper age limit and pre-stroke disability cap as part of clinical trial enrolment criteria disproportionately excludes females from the study. Even if an equal number of males and females of similar ages are included in the study, the female patients in the study will not be sufficiently representative of the general female stroke population. mRS, modified Rankin Scale.
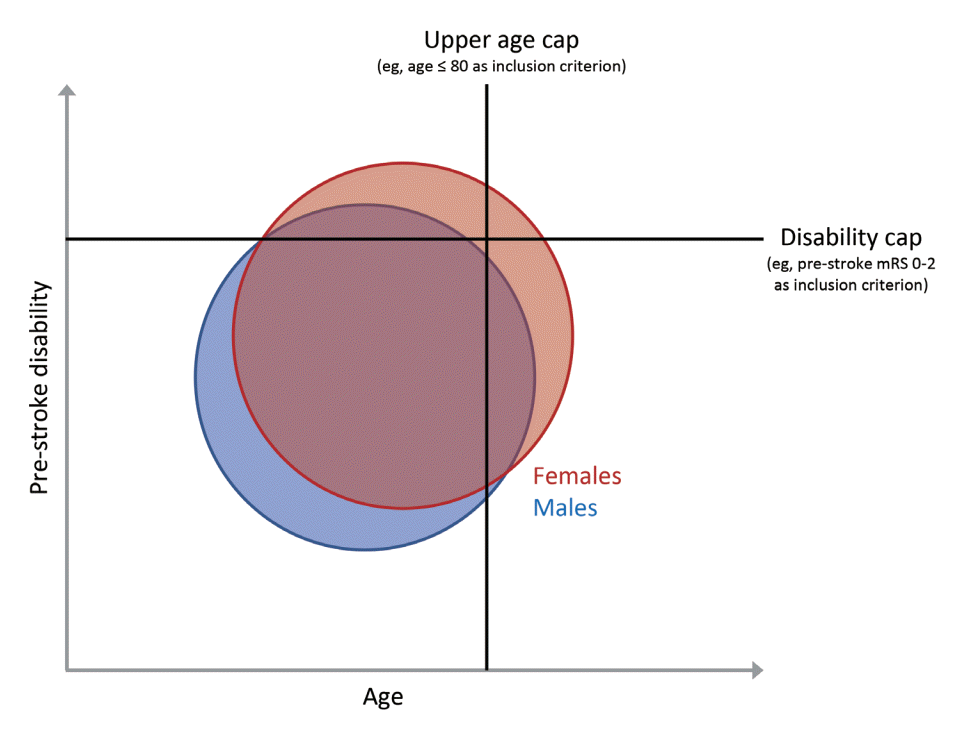
Table 1.
Key sex- and gender-related differences in stroke patients’ baseline status
| Variables | Differences in baseline status | |
|---|---|---|
| Biological (sex-related) | ||
| Age | Female acute ischemic stroke (AIS) patients are approximately 5 years older than males [9]. There may also be “interaction-type” effects whereby in certain ethnic groups, stroke risk seems higher in elderly women, but not in men [43]. | |
| Pre-stroke disability | Female AIS patients are more often dependent in their daily activities prior to their stroke than men (partially explained by their older age at stroke onset) [44] | |
| Stroke etiology | Female AIS patients more often suffer from atrial fibrillation prior to their stroke, and as a result, female strokes are more often cardioembolic in origin [15]. There also seems to be a stronger association between hypertension and diabetes, particularly type 1 diabetes, in women compared with men [6]. Peripheral vascular disease as an AIS risk factor is more common in male AIS patients [16]. | |
| Prior medication | Despite the higher prevalence of atrial fibrillation in female stroke patients, proportions of patients treated with anticoagulant therapy prior to the stroke does not differ between men and women [16], and aspirin intake prior to the stroke is even less common in female patients [15]. | |
| Obesity | Obesity is associated with an elevated risk of stroke in women and men, but this association is stronger in women [45]. | |
| Hypertension | The association between hypertension and the risk of stroke is higher in women compared to men, even after adjustment for use of antihypertensive medication [45-47]. | |
| Hormonal factors | Endogenous and exogenous hormone imbalances (e.g., due to oral contraception) as well as pregnancy-related changes in pathophysiology are stroke risk factors unique to women and are discussed comprehensively elsewhere [48]. | |
| Sociocultural (gender-related) | ||
| Social network | Female AIS patients are more likely to be widowed and live alone at the time of stroke onset [11-14] | |
| Socioeconomic status | Only few and inconsistent data are available on whether the impact of socioeconomic status on stroke risk and outcomes differs between men and women. Two European studies found increased risk of recurrent in women with lower annual income, but not in men [45,49]. On the other hand, decreased income was associated with increased case-fatality in men but not women [49]. | |
| Smoking | Smoking as an AIS risk factor is encountered more often in male AIS patients [16] At the same time, the association of smoking and any stroke is greater in women than in men [45]. | |
| Alcohol overuse | Alcohol overuse as an AIS risk factor is encountered more often in male AIS patients [16]. | |
Table 2.
Key points on sex- and gender-related aspects in acute stroke care




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download