Abstract
Purpose
Materials and Methods
Results
Conclusion
Electronic Supplementary Material
Notes
Ethical Statement
A total of six institutes participated in the ring trial and analyses were conducted on four different NGS panels. This retrospective cohort study was approved by the Institutional Review Board of each institution (institution A: 2020-05-095; institution B: H-2009-056-1155; Institution C: KC20SIDI0651; institution D: 2020-1386; institution E: 4-2020-0721; institution F: KUMC 2020-09-031) and performed in accordance with the principles of the Declaration of Helsinki. The requirement for informed consent was waived.
Author Contributions
Conceived and designed the analysis: Lee SE, Lee MS, Choi YL.
Collected the data: Lee SE, Lee MS, Choi YL.
Contributed data or analysis tools: Lee SE, Lee MS, Jeon YK, Shim
HS, Kang J, Kim J, Choi YL.
Performed the analysis: Lee SE, Lee MS, Jeon YK, Shim HS, Kang J, Kim J, Choi YL.
Wrote the paper: Lee SE, Lee MS, Kim J, Choi YL.
ACKNOWLEDGMENTS
References
Fig. 1
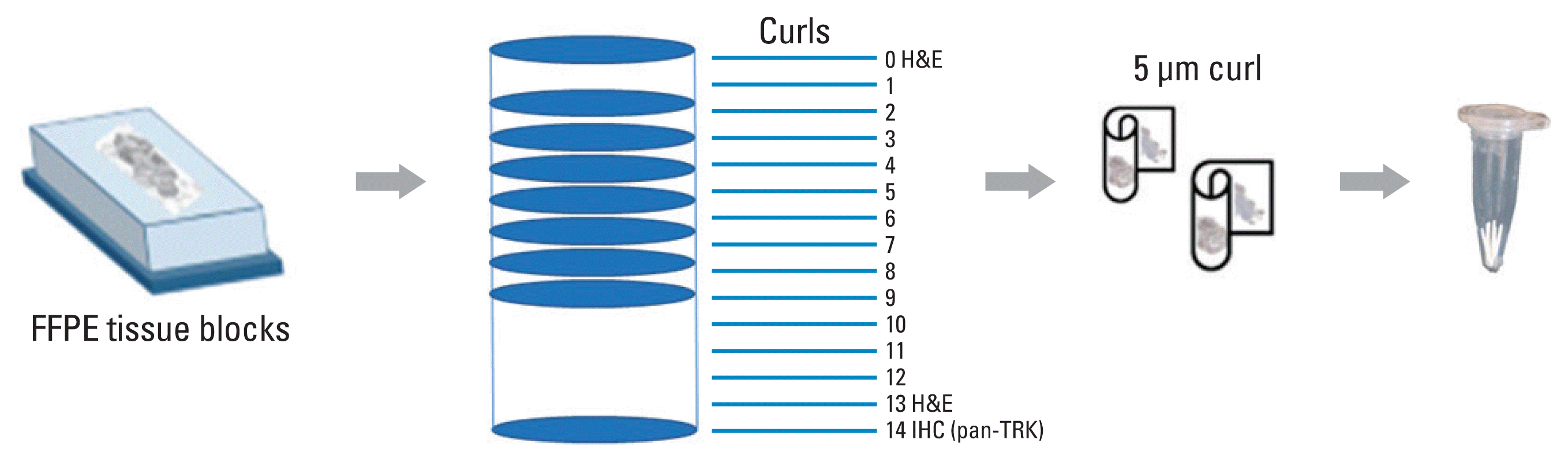
Fig. 2

Table 1
Table 2
Table 3
| Sample ID | Fusion type | Exon No. of BP | Supporting read count | Inframe/Out-of-frame |
|---|---|---|---|---|
| BN01 | ETV6-NTRK3 | E(5)N(15) | 9 | Inframe |
|
|
||||
| BN03a) | TPR-NTRK1 | T(4)N(10) | 128 | Inframe |
|
|
||||
| BN04 | ETV6-NTRK3 | E(5)N(15) | 24 | Inframe |
| E(5)N(14) | 15 | Intronic fusion | ||
| E(6)N(14) | 17 | Inframe | ||
|
|
||||
| BN07 | TPM3-NTRK1 | T(7)N(10) | 80 | Inframe |
|
|
||||
| BN10a) | HOOK3-NTRK2 | H(13)N(14) | 290 | Inframe |
| H(2)N(14) | 81 | Out-of-frame | ||
| H(6)N(14) | 6 | Inframe | ||
| H(2)N(14) | 71 | Inframe | ||
|
|
||||
| BN11 | TPM3-NTRK1 | T(int7)N(8) | 29 | Intronic fusion |
| T(7)N(10) | 524 | Inframe | ||
|
|
||||
| BN13 | RBPMS-NTRK3 | R(5)N(14) | 939 | Inframe |
|
|
||||
| BN17 | TPM3-NTRK1 | T(7)N(10) | 1,269 | Inframe |
| T(8)N(10) | 346 | Out-of-frame | ||
Table 4
| Sample ID | Fusion type | Fusion supporting reads counts (curl number of sample) | |||||
|---|---|---|---|---|---|---|---|
| A (TSO 500) | B (Lung Cancer Panel v3.0) | C (OCA v3) | D (OncoPanel v.4.3) | E (TSO 500) | F (OCA v3) | ||
| BN01 | ETV6-NTRK3 | Not detected (1, 2) | 12 (3, 4) | 2,082 (7, 8) | Not detected (5, 6) | 7 (9, 10) | Not detected (11, 12) |
| BN02 | - | - | - | - | - | - | - |
| BN03 | TPR-NTRK1 (128) | 128 (1, 2) | 406 (3, 4) | 36,852 (7, 8) | 37 (5, 6) | 172 (9, 10) | 94,492 (11, 12)a) |
| BN04 | ETV6-NTRK3 (24) | 24 (9, 10) | 8 (3, 4) | QC fail (1, 2) | Not detected (11, 12) | 122 (5, 6) | 218,059 (11, 12) |
| BN05 | - | - | - | - | - | - | - |
| BN06 | - | - | - | - | - | - | - |
| BN07 | TPM3-NTRK1 (80) | 80 (9, 10) | 54 (11, 12) | QC fail (1, 2) | 13 (3, 4) | 321 (5, 6) | 447,970 (7, 8) |
| BN08 | - | - | - | - | - | - | - |
| BN09 | - | - | - | - | - | - | - |
| BN10 | HOOK3-NTRK2 (290) | 290 (5, 6) | 220 (7, 8) | Not covered (11, 12) | Not detected (9, 10) | 795 (3, 4) | Not covered (1, 2) |
| BN11 | TPM3-NTRK1 (553) | 524 (3, 4) | 929 (5, 6) | 612,303 (7, 8) | 16 (1, 2) | 1,309 (9, 10) | 302,550 (11, 12) |
| BN12 | - | - | - | - | - | - | - |
| BN13 | RBPMS-NTRK3 (939) | ? (9, 10) | 155 (3, 4) | 2,569,146 (7, 8) | Not detected (5, 6) | 1,390 (1, 2) | 530,524 (9, 10) |
| BN14 | - | - | - | - | - | - | - |
| BN15 | - | - | - | - | - | - | - |
| BN17 | TPM3-NTRK1 (1,615) | 1,269 (1, 2) | 251 (11, 12) | 744,925 (11, 12) | 110 (7, 8) | 2,843 (3, 4) | 21,468 (5, 6) |
| BN18 | - | - | - | - | - | - | - |
Table 5
| Fusion Supporting Reads counts | ||||||
|---|---|---|---|---|---|---|
| A (TSO 500) | B (Lung Cancer Panel v3.0) | C (OCA v3) | D (OncoPanel v.4.3) | E (TSO 500) | F (OCA v3) | |
| TPM3-NTRK1 | 234 | 44 | 39,211 | NA | 278 | 21,468 |
| LMNA-NTRK1 | 324 | 29 | - | NA | 426 | - |
| IRF2BP2-NTRK1 | 188 | 35 | 112 | NA | 341 | - |
| TFG-NTRK1 | 184 | 14 | 46,023 | NA | 174 | 114,933 |
| SQSTM1-NTRK1 | 266 | 31 | 7,200 | NA | 418 | 33a) |
| AFAP1-NTRK2 | 133 | 53 | 17,371 | NA | 205 | 16a) |
| QKI-NTRK2 | 191 | 5 | 38,997 | NA | 229 | 1,611,524 |
| TRIM24-NTRK2 | 163 | - | 5,791 | NA | 172 | 28a) |
| NACC2-NTRK2 | 168 | - | 16,377 | NA | 199 | 743,802 |
| PAN3-NTRK2 | 152 | 32 | NC | NA | 320 | NC |
| ETV6-NTRK3 (E5N14) | 147 | 70 | 28,094 | NA | 268 | 489 |
| ETV6-NTRK3 (E5N15) | 97 | 68 | 16,823 | NA | 224 | 1,955 |
| ETV6-NTRK3 (E4N15) | 153 | 18 | 1,024 | NA | 194 | 3a) |
| ETV6-NTRK3 (E4N14) | 192 | 16 | 2,391 | NA | 212 | 4a) |
| BTBD1-NTRK3 | 100 | 58 | 40,086 | NA | 167 | 29,051 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download